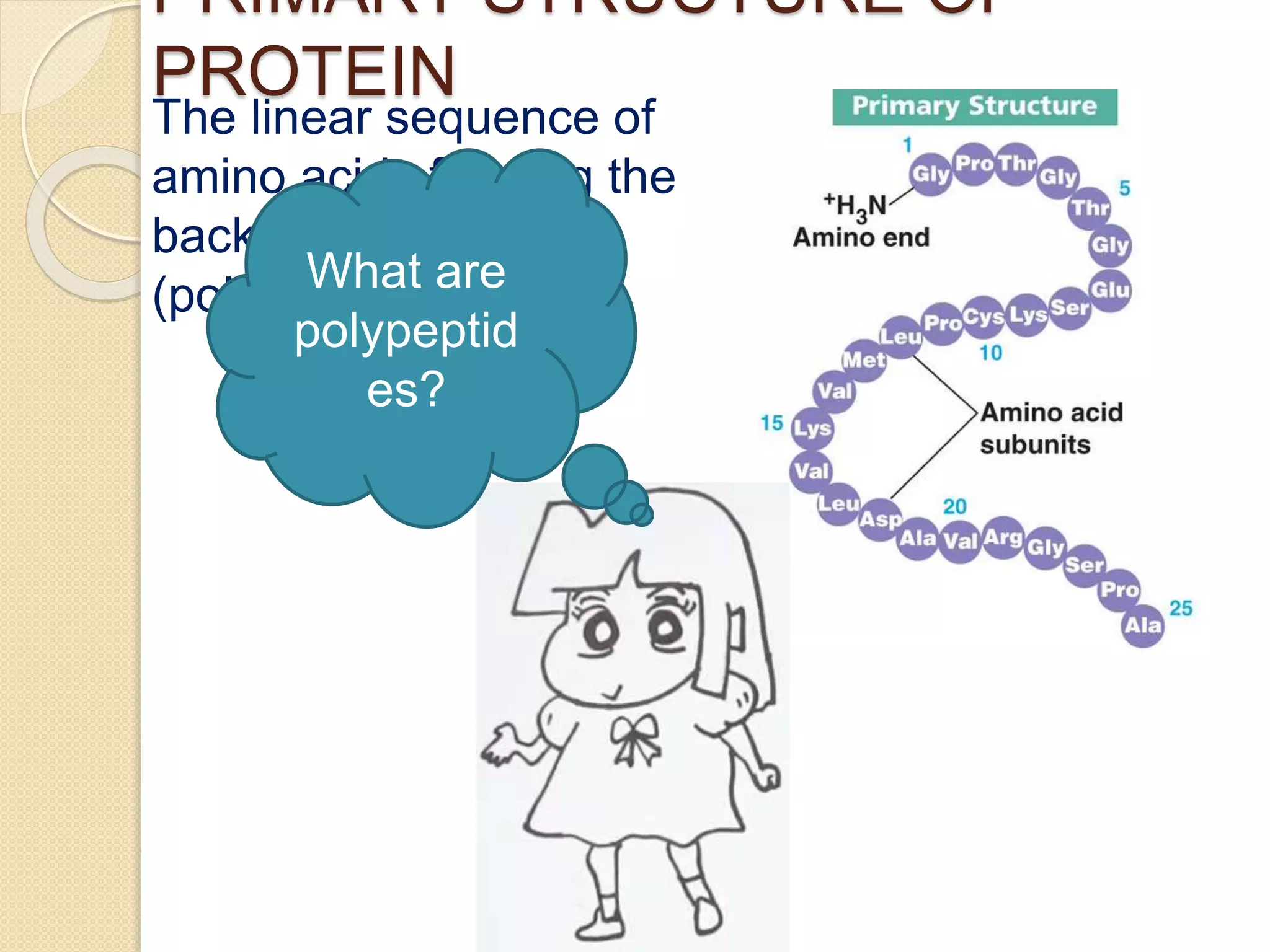
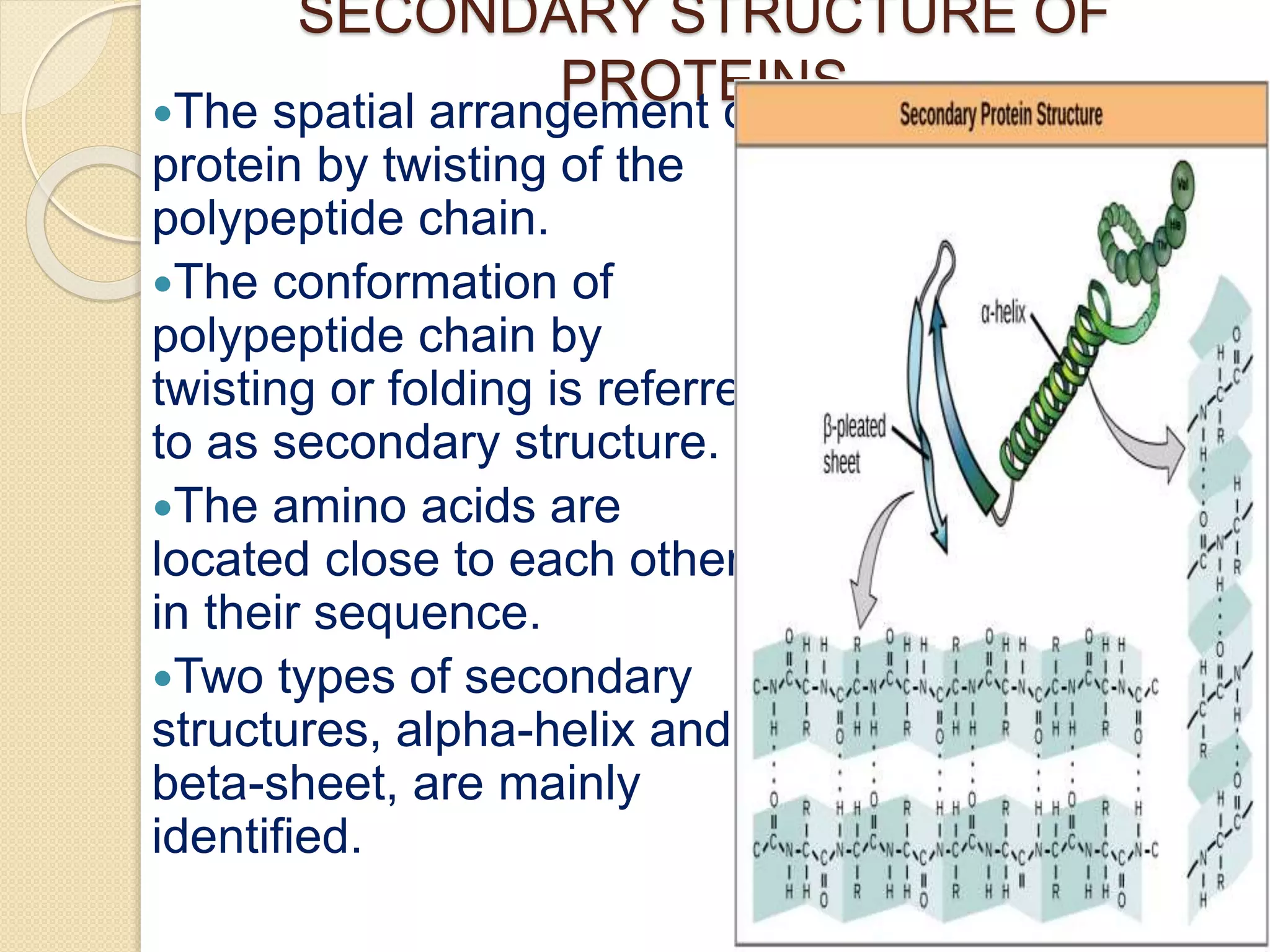
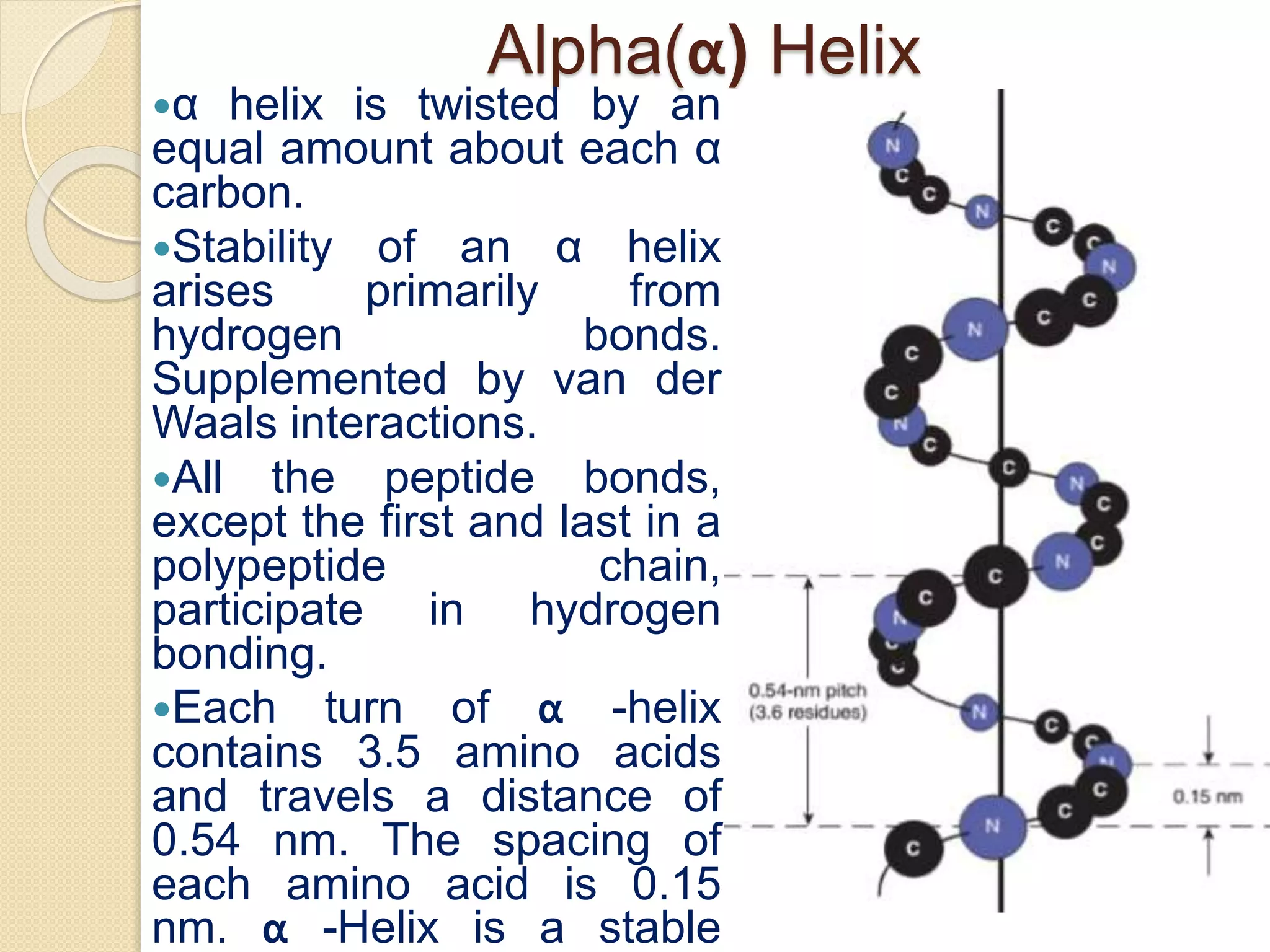
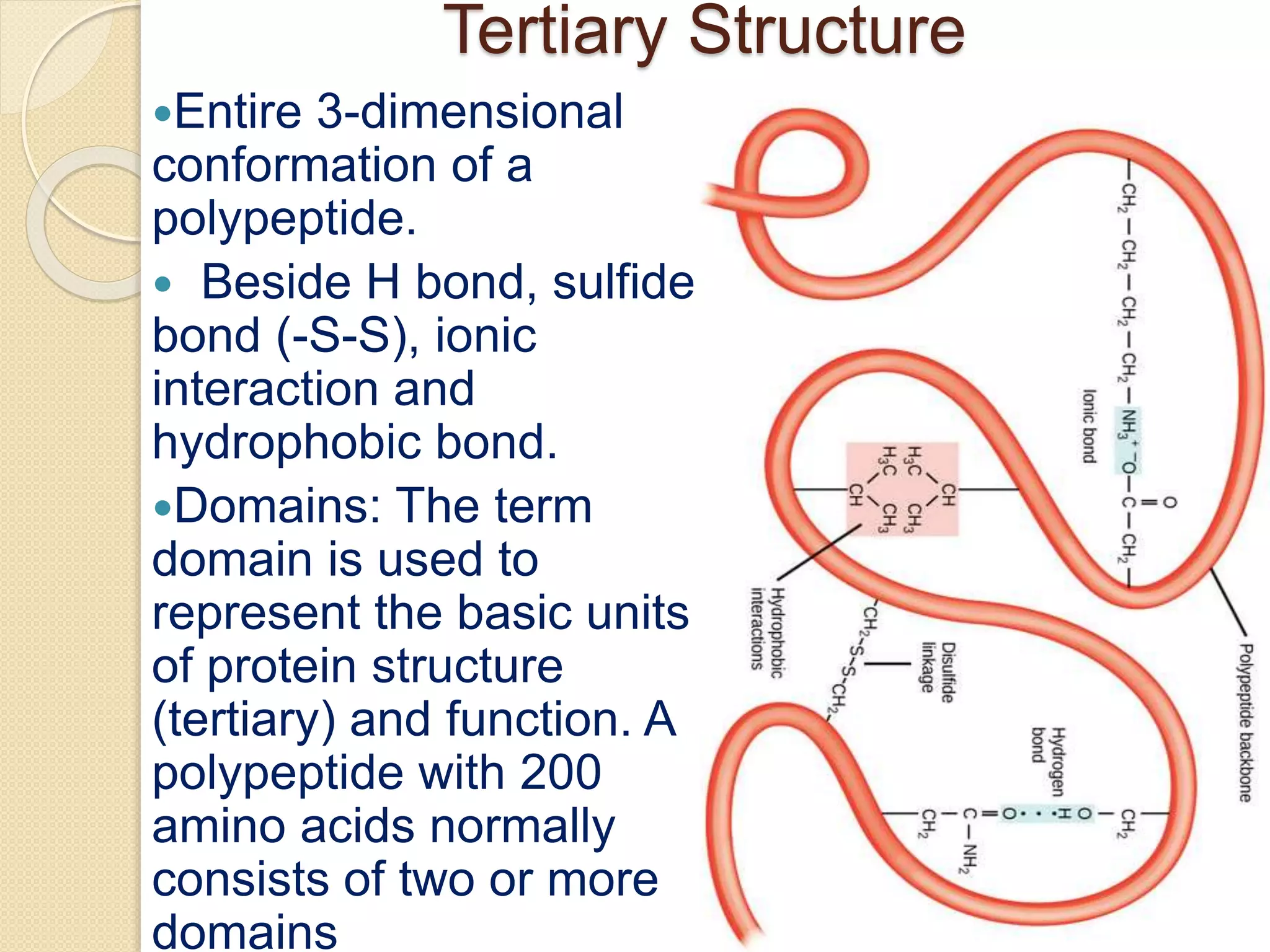
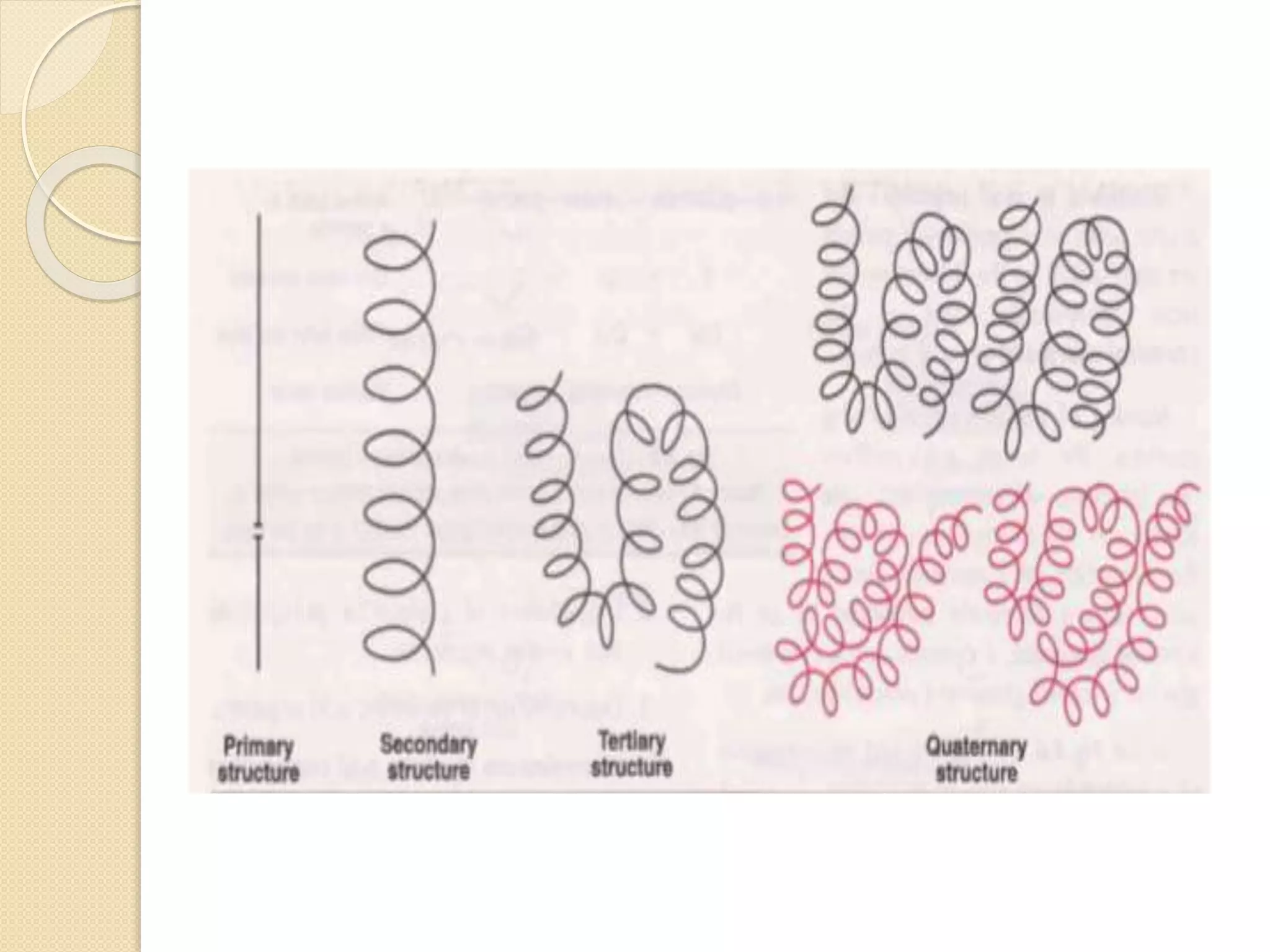
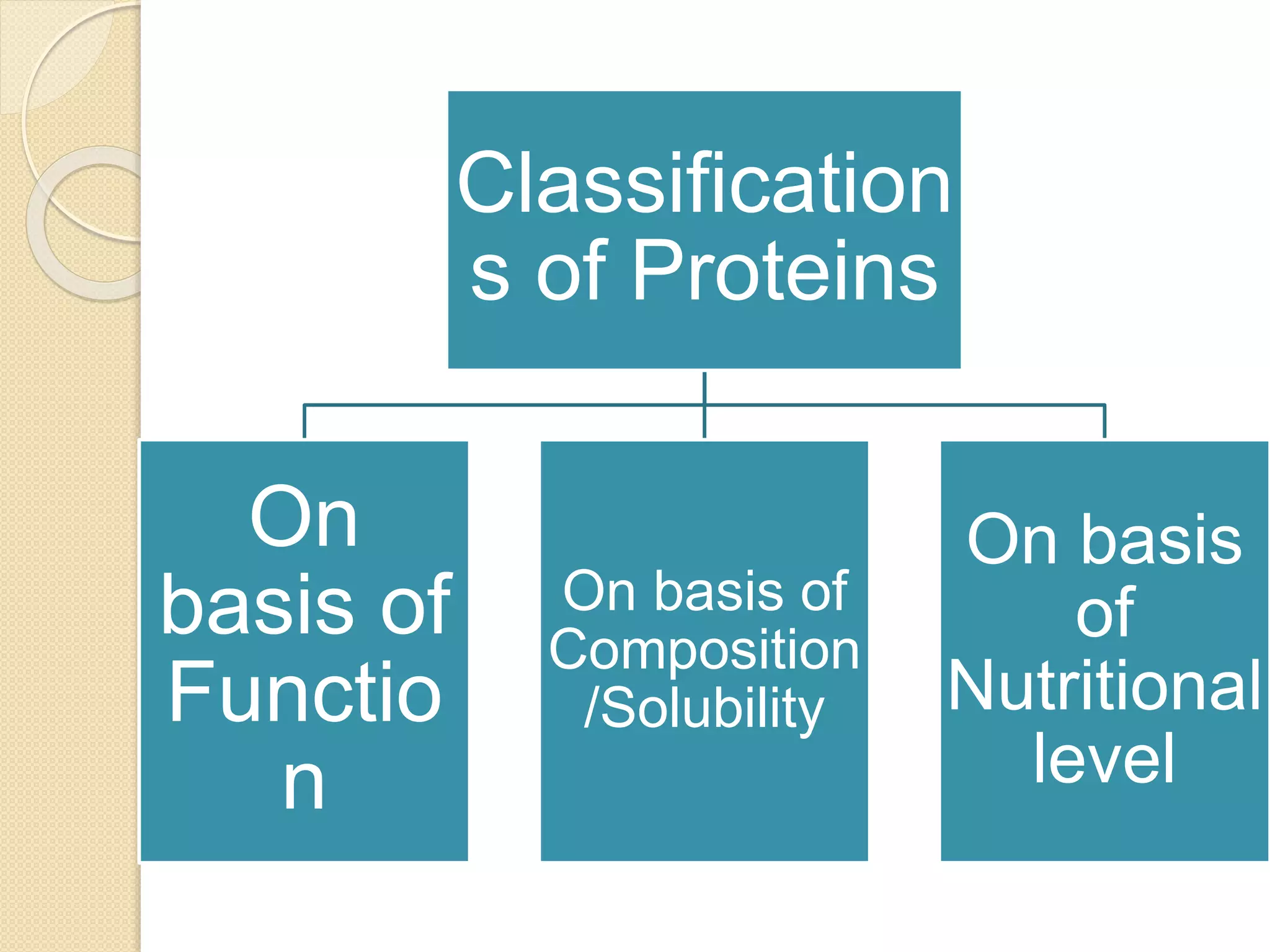
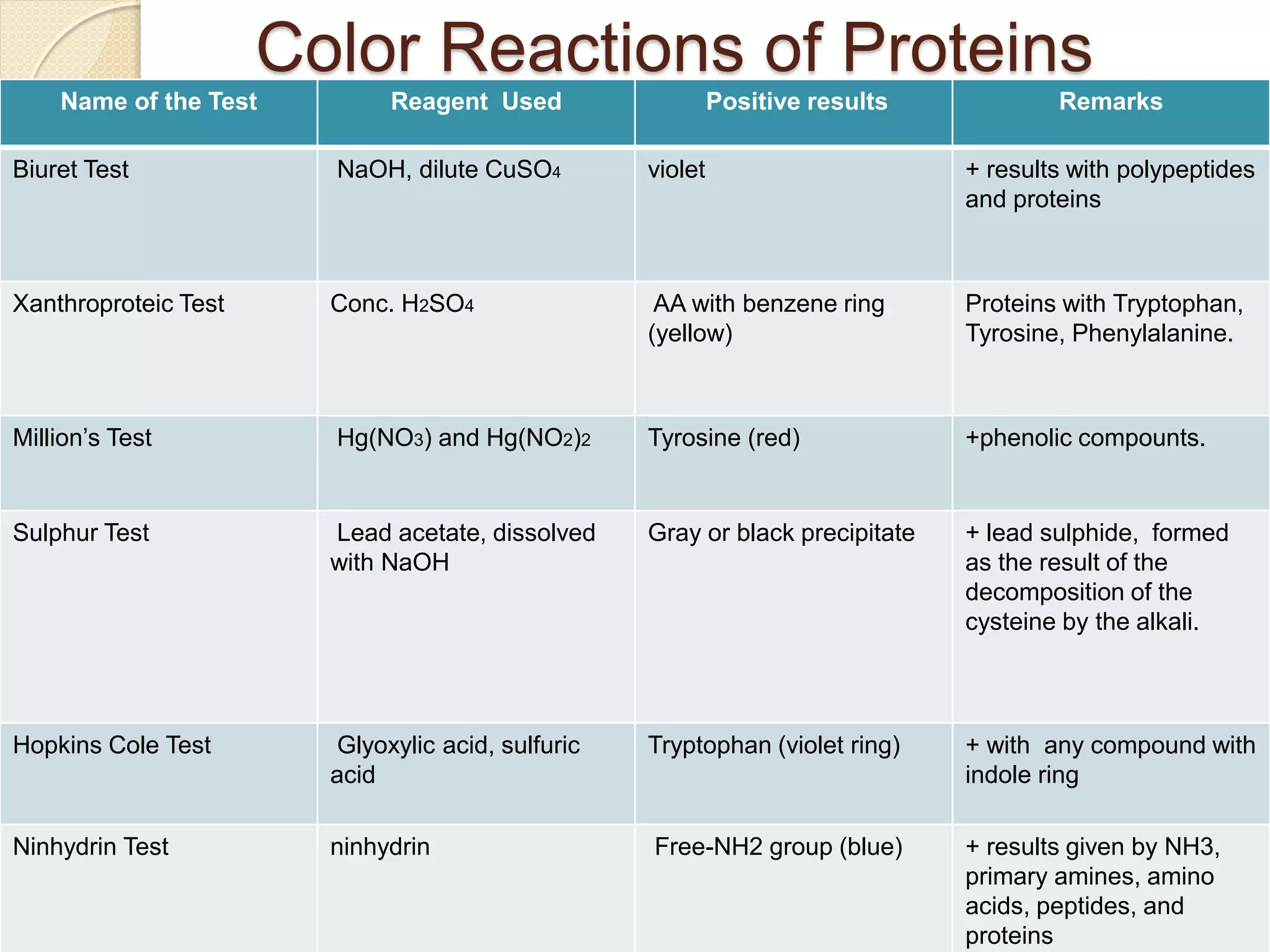
This document discusses the structure and classifications of proteins. It describes the primary, secondary, tertiary, and quaternary structures of proteins. The primary structure is the linear sequence of amino acids. Secondary structures include alpha-helices and beta-sheets formed by hydrogen bonding between amino acids. Tertiary structure refers to the 3D conformation of the entire polypeptide chain. Quaternary structure involves interactions between multiple polypeptide subunits. The document also classifies proteins based on their function, composition/solubility, and nutritional levels. Common protein structures and several color reaction tests for identifying proteins are outlined.