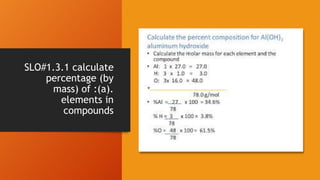
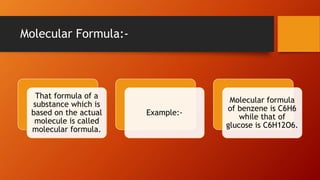
1. Stoichiometry is the quantitative relationship between reactants and products in a balanced chemical equation. It allows calculations of mass, moles, particles, and volume from a balanced equation under specified conditions.
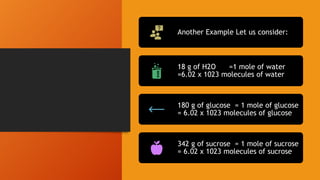
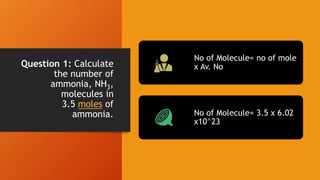
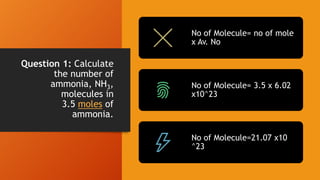
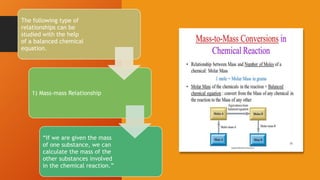
2. Calculations include mass-mass, mass-mole, mass-volume, and mole-mole relationships between substances. Conversions use mole ratios as conversion factors.
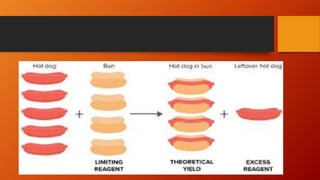
3. Limiting reagent refers to the reactant that is completely used up first and thus limits the amount of product that can be formed, analogous to the relationship between kababs and bread slices in making sandwiches.