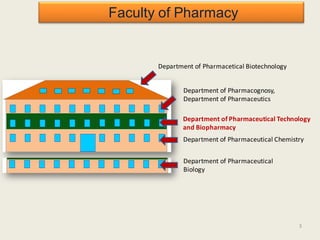
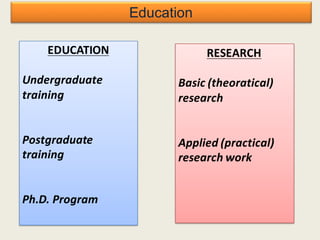
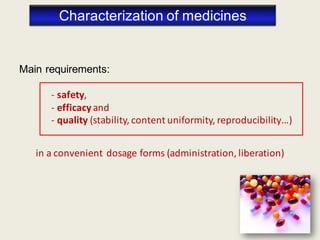
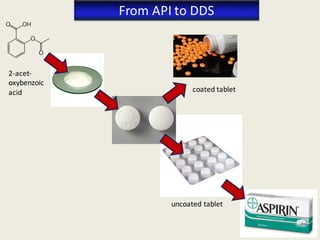
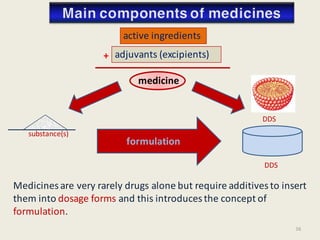
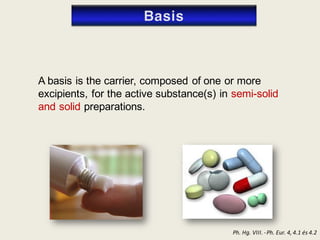
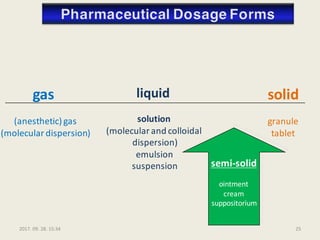
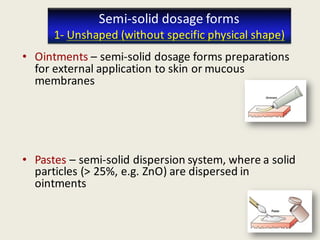
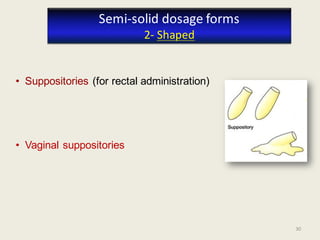
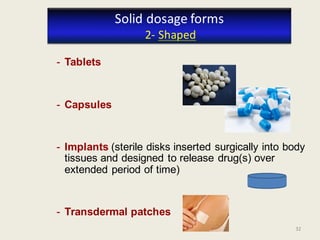
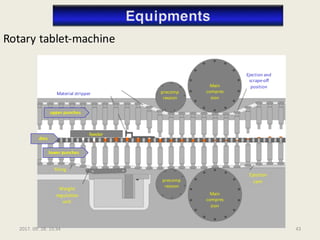
The document outlines the requirements for acceptance in the Pharmaceutical Technology and Biopharmacy program at the University of Pécs, including attendance, assessment performance, and rules regarding absences. It provides an overview of the types and classification of pharmaceutical preparations, emphasizing the importance of safety, efficacy, and quality in medication formulation. The document also covers various dosage forms and the role of excipients, illustrating the complexities involved in pharmaceutical development and manufacturing.