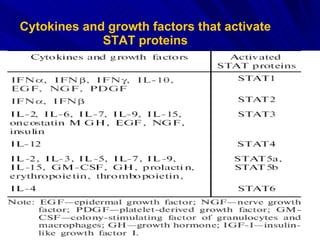
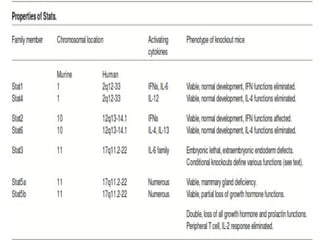
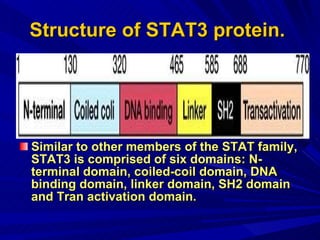
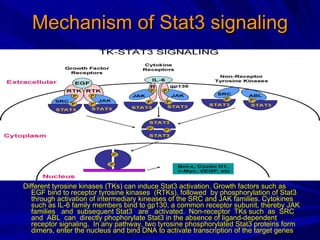
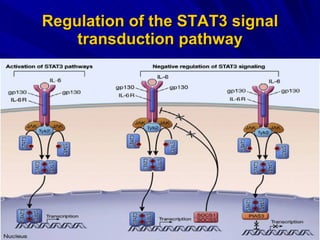
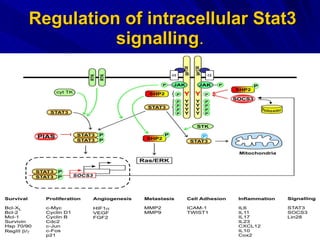
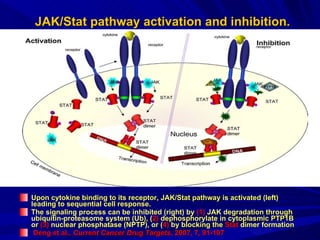
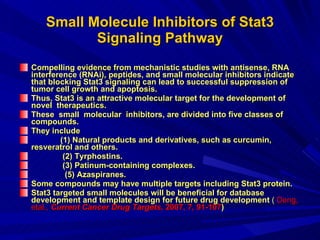
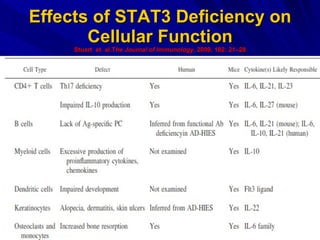
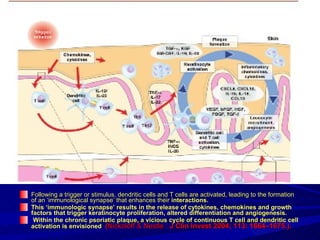
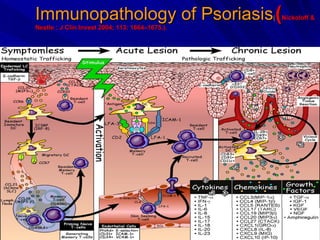
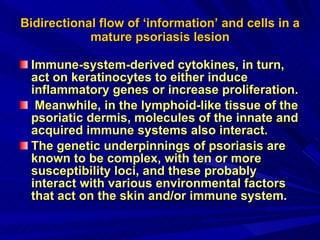
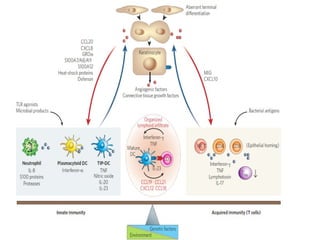
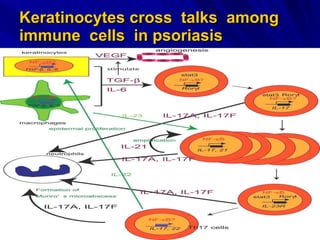
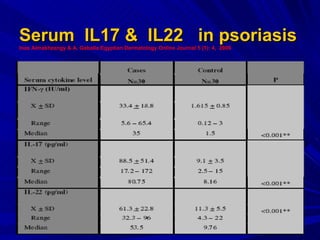
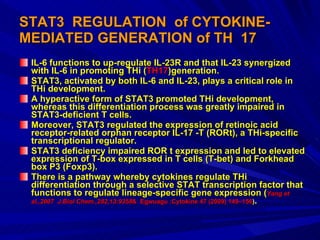
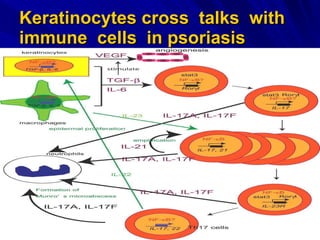
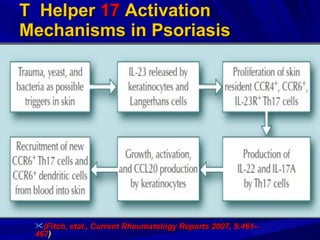
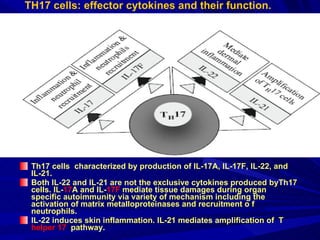
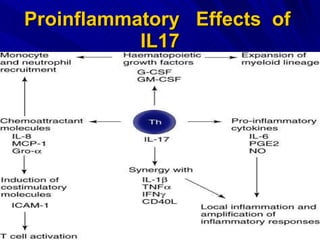
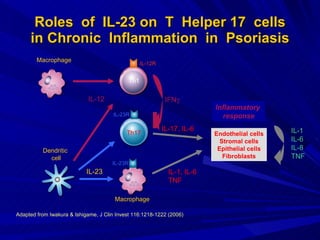
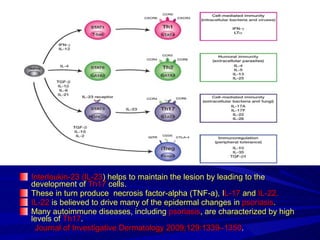
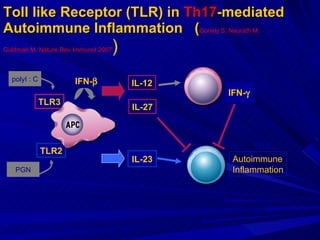
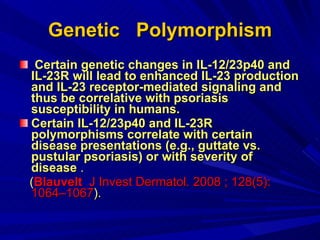
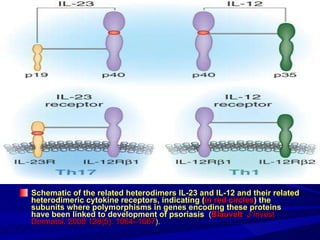
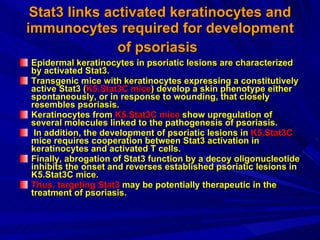
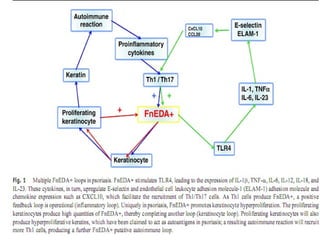
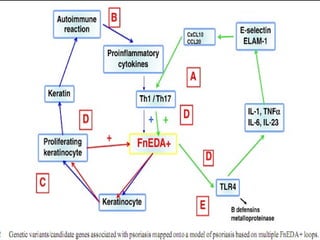
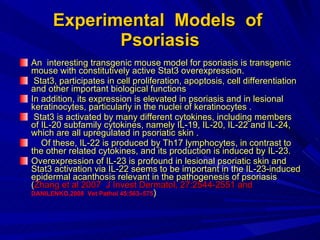
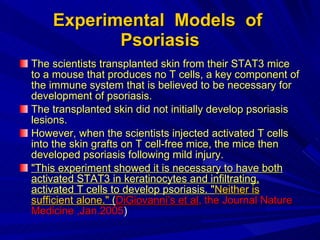
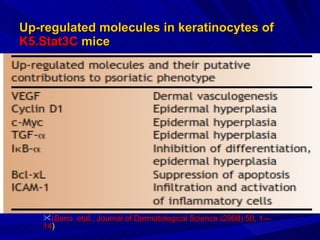
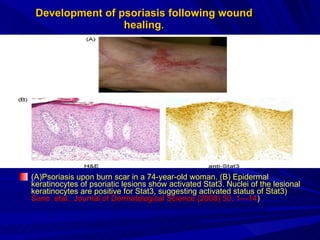
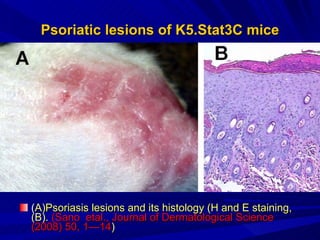
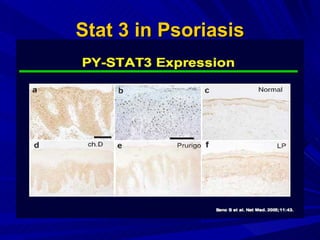
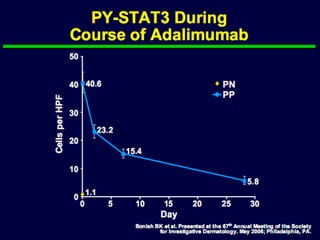
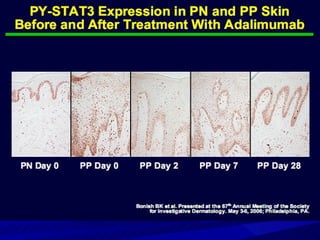
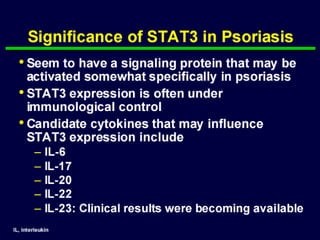
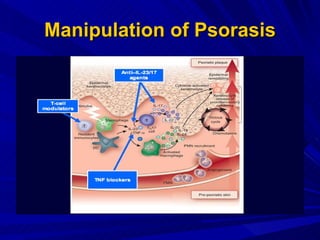
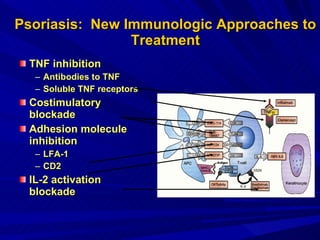
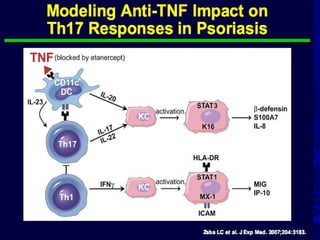
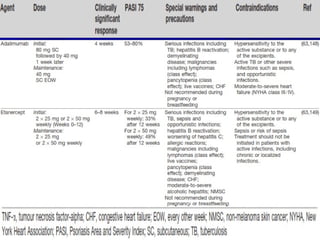
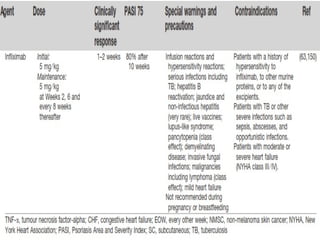
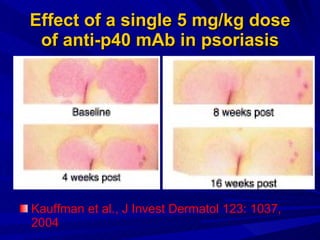
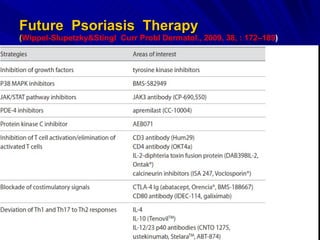
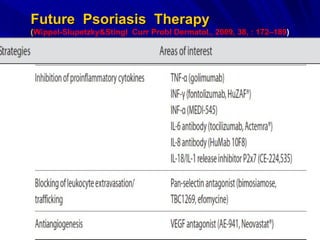
STAT3 protein plays a role in psoriasis pathogenesis through its involvement in immune cell signaling. STAT3 is activated by cytokines and growth factors and acts as a signal transducer downstream of cytokine receptors. In psoriasis, STAT3 signaling in immune cells such as T cells and dendritic cells leads to the release of inflammatory cytokines that trigger keratinocyte proliferation and skin inflammation. The interaction between immune cells and keratinocytes forms a positive feedback loop that maintains the chronic psoriatic plaques.