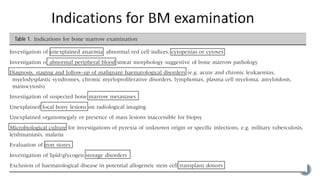
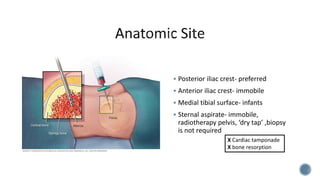
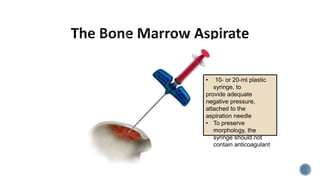
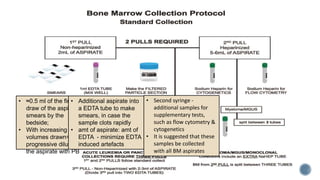
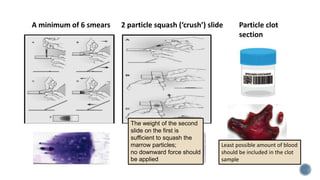
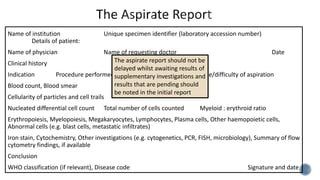
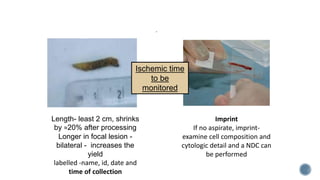
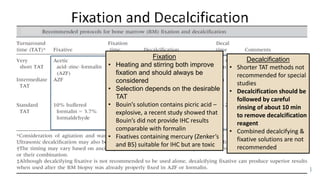
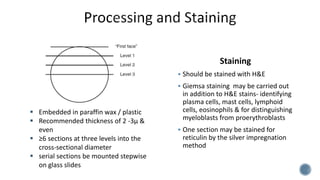
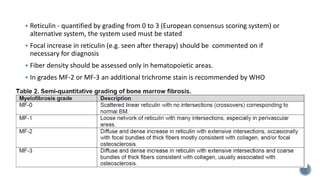
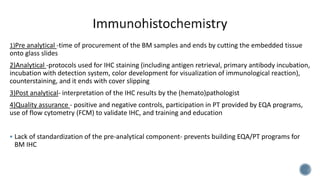
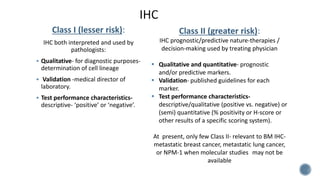
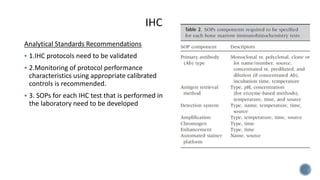
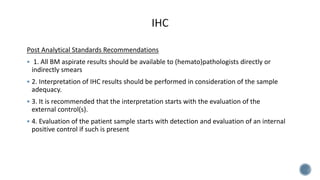
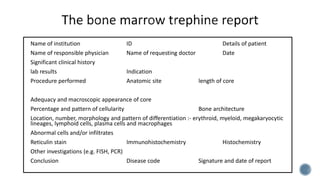
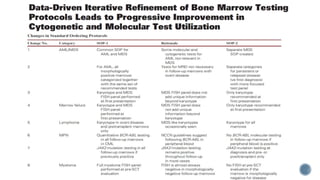
This document provides guidelines for standardizing bone marrow examination and reporting. It discusses specimen collection, processing, analysis and reporting of bone marrow aspirates and biopsies. Uniform procedures are important for accurate diagnosis and classification of blood and bone marrow disorders. The guidelines cover indications, techniques, analyses including morphology, immunohistochemistry and special stains, as well as turnaround times and quality assurance. Adhering to these standardized procedures and reporting elements can improve completeness and consistency of bone marrow examination across institutions.