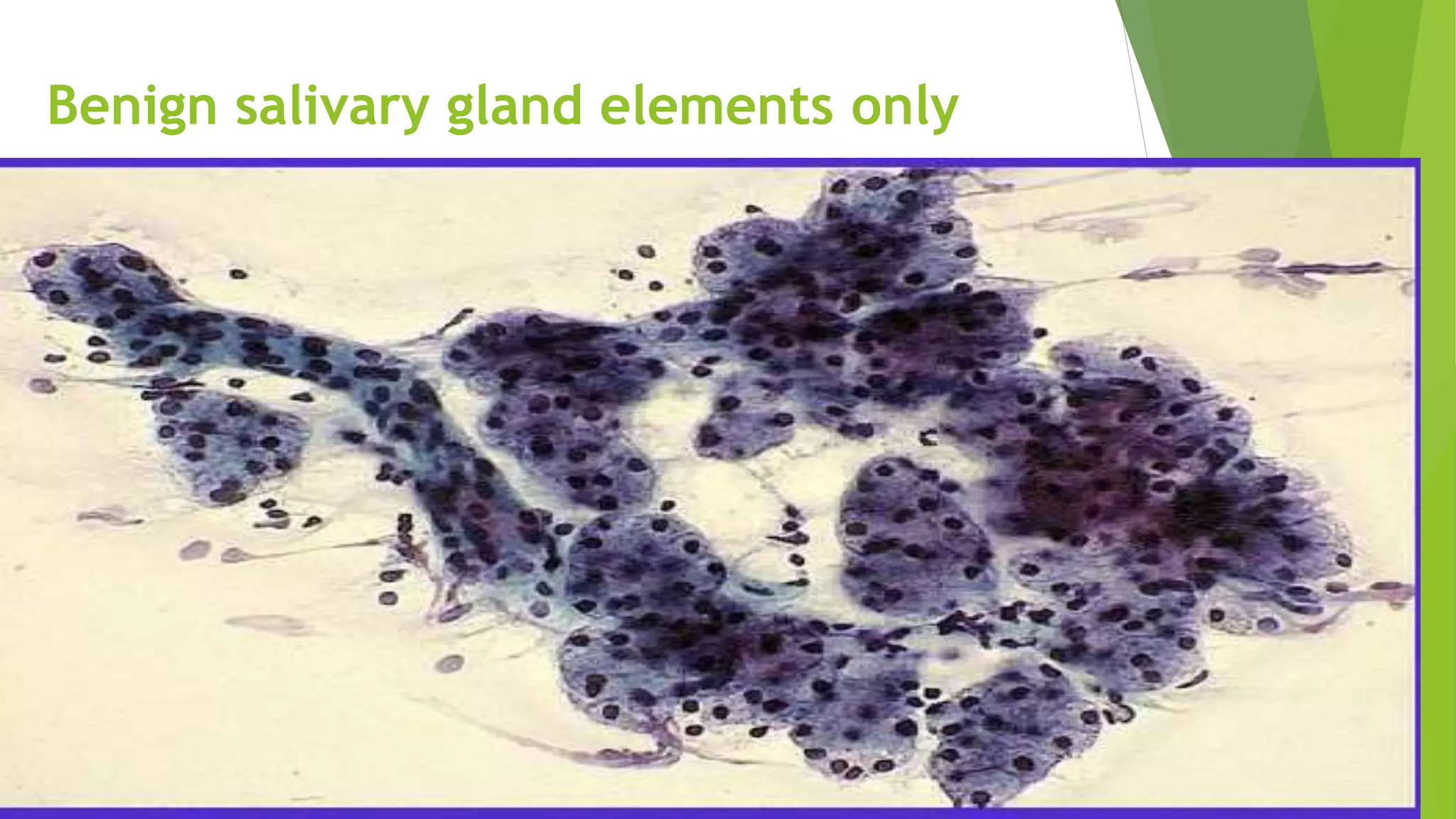
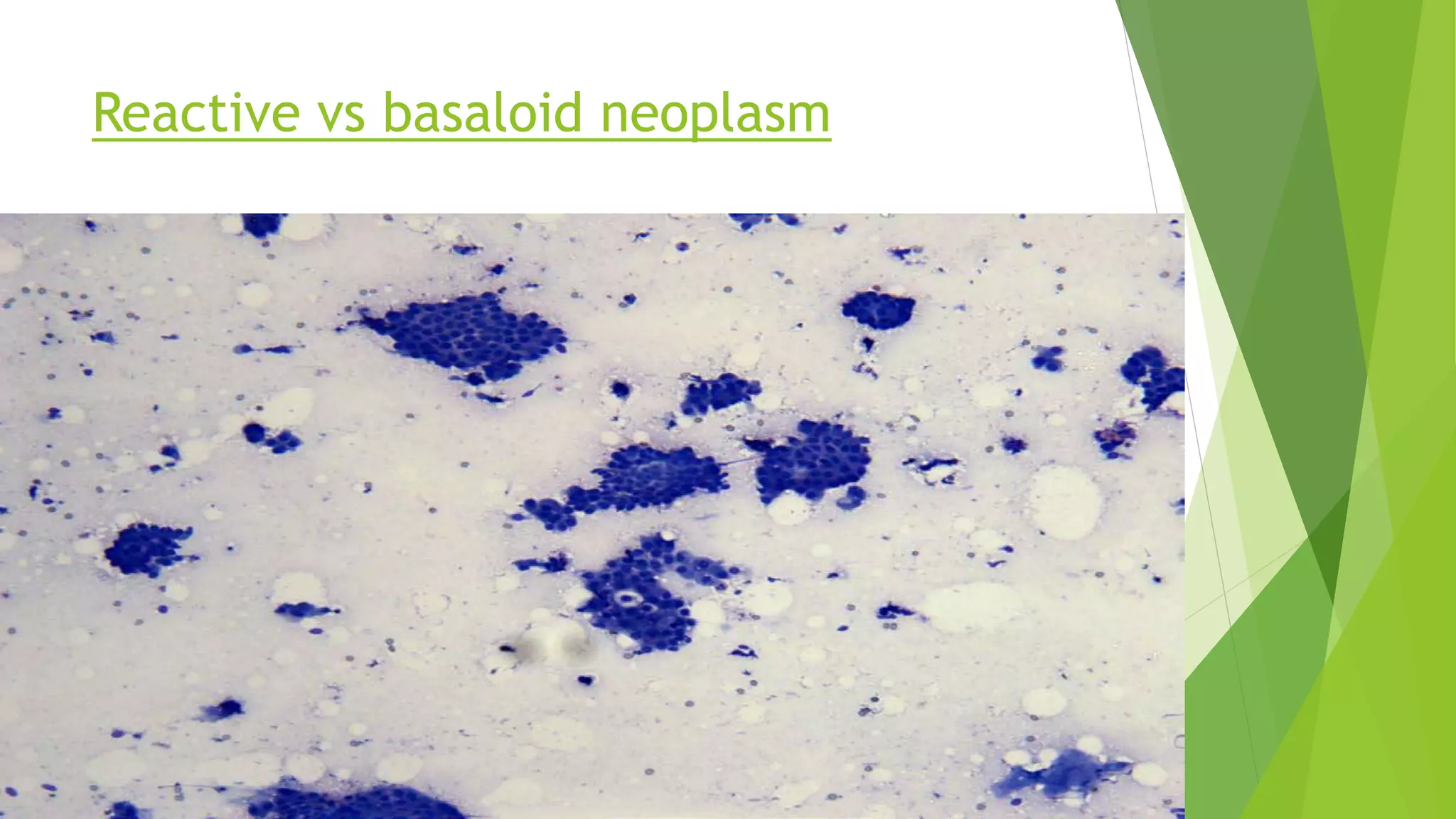
The document discusses the development and benefits of the Milan System for Reporting Salivary Gland Cytopathology. It aims to standardize terminology for salivary gland FNA reports which previously lacked uniformity. The system categorizes specimens as non-diagnostic, non-neoplastic, atypia of undetermined significance, neoplastic (benign or uncertain malignant potential), suspicious for malignancy, or malignant. It is intended to improve communication between pathologists and clinicians, enhance patient care, and facilitate research by allowing standardized data collection across institutions. While validation is ongoing, the system provides a practical framework for uniform reporting of salivary gland cytology.