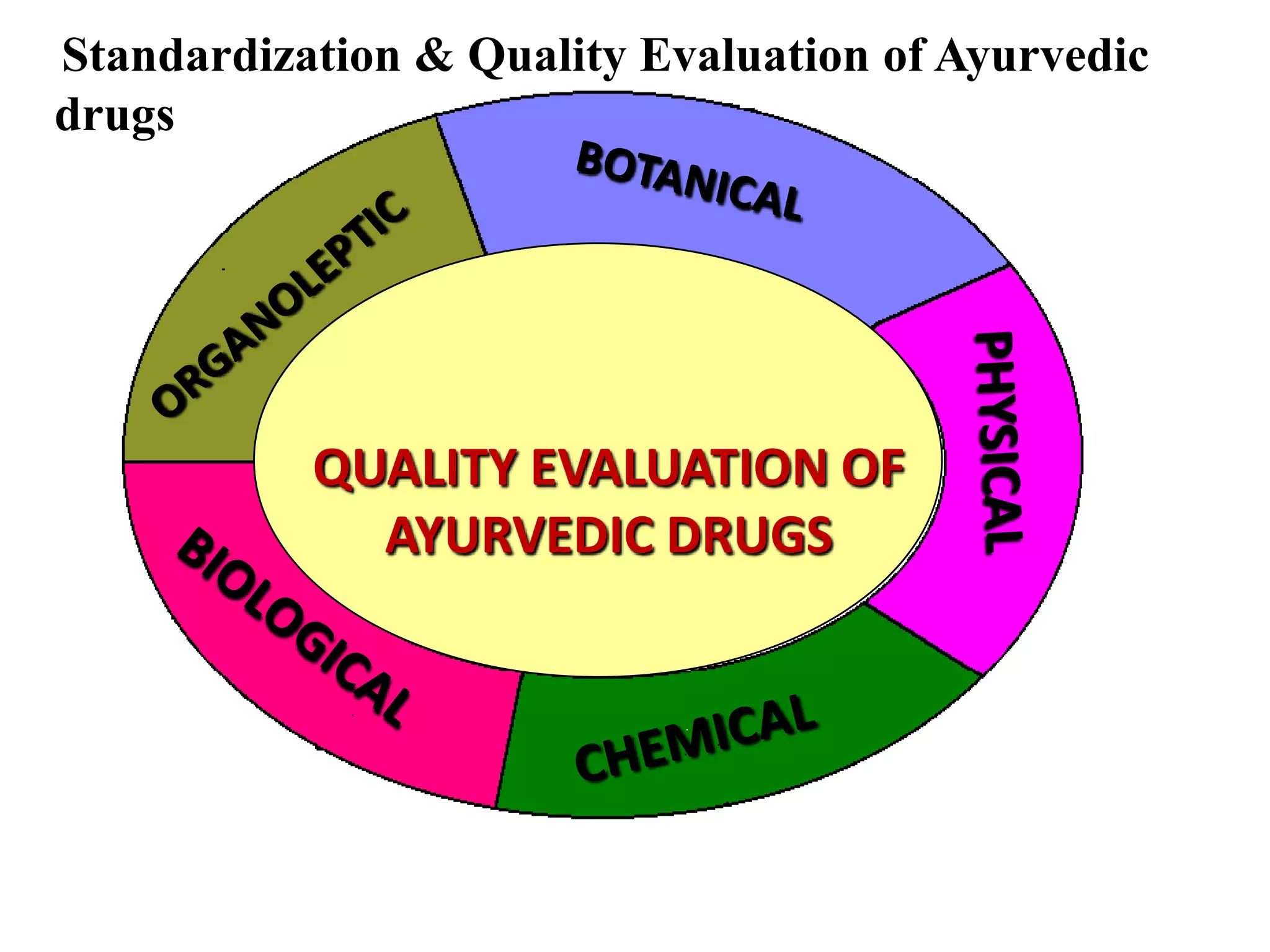
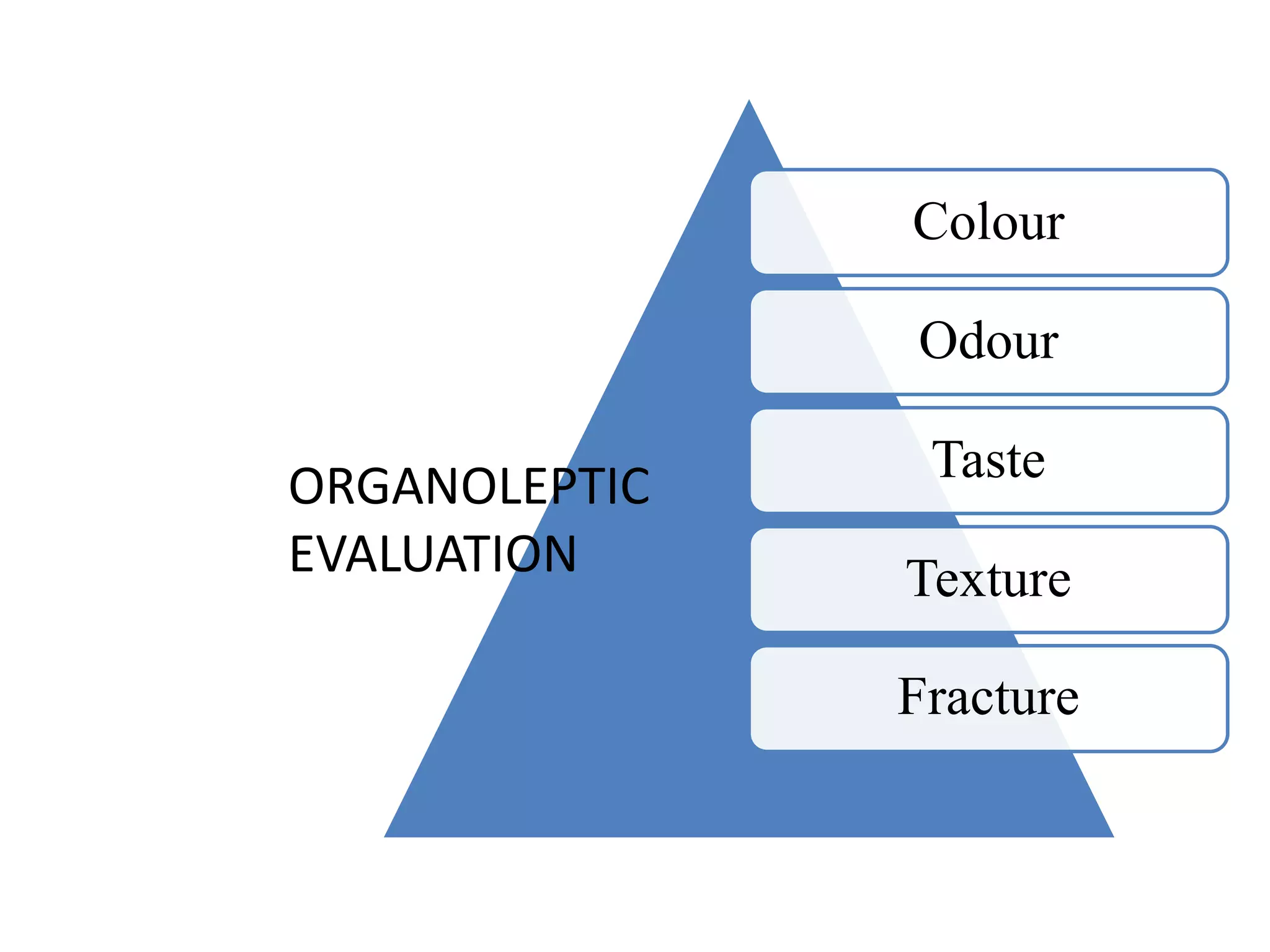
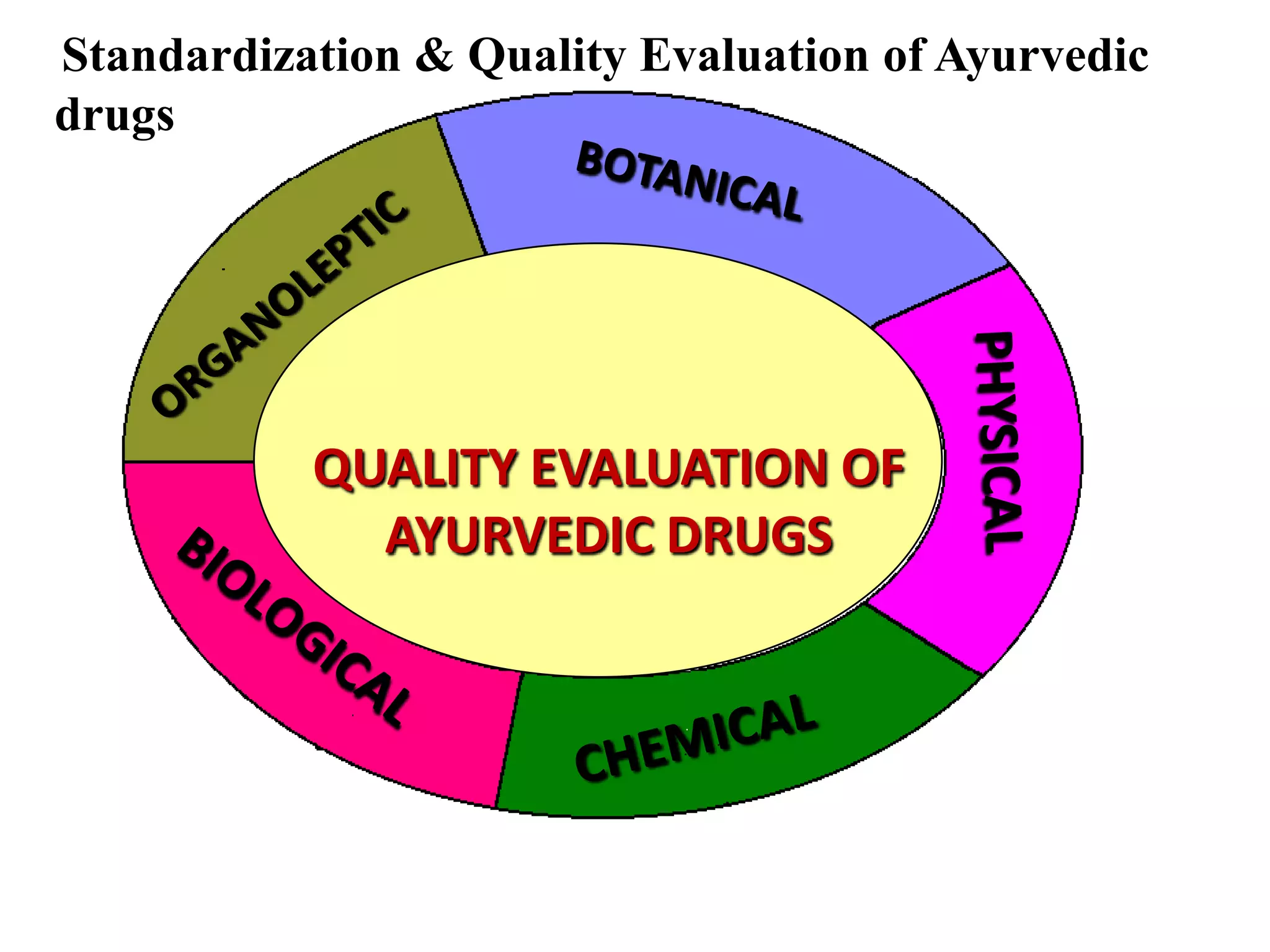
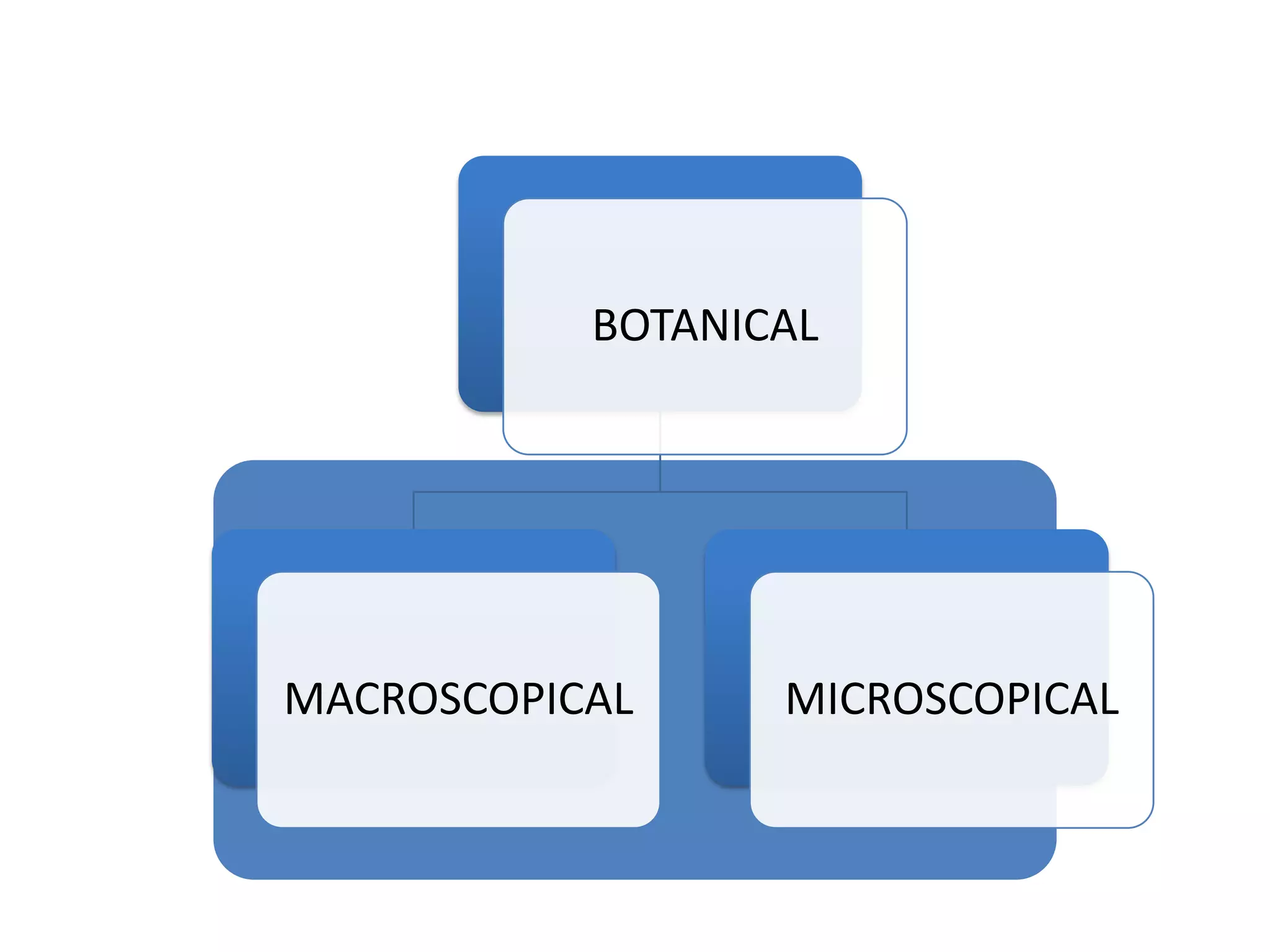
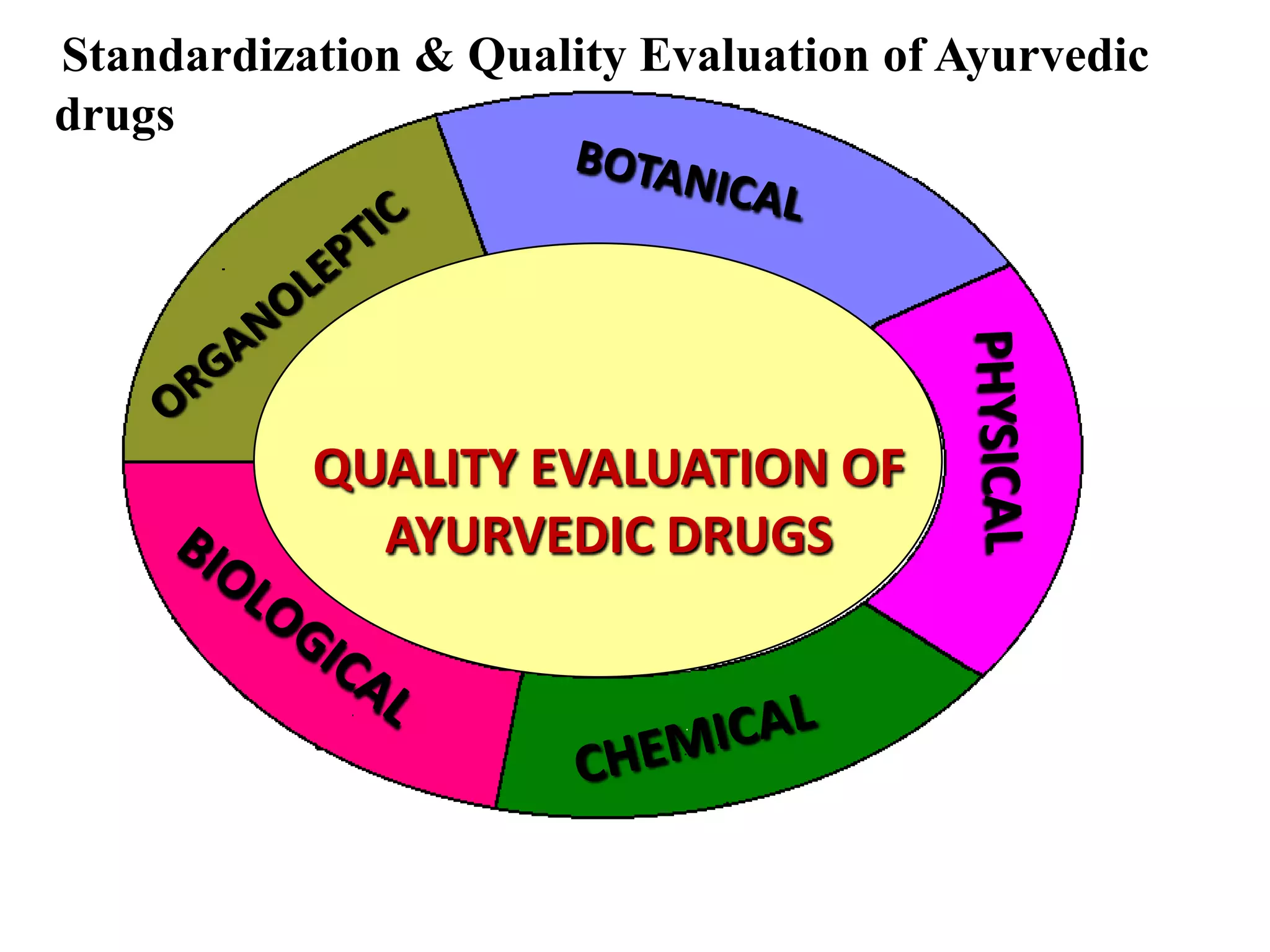
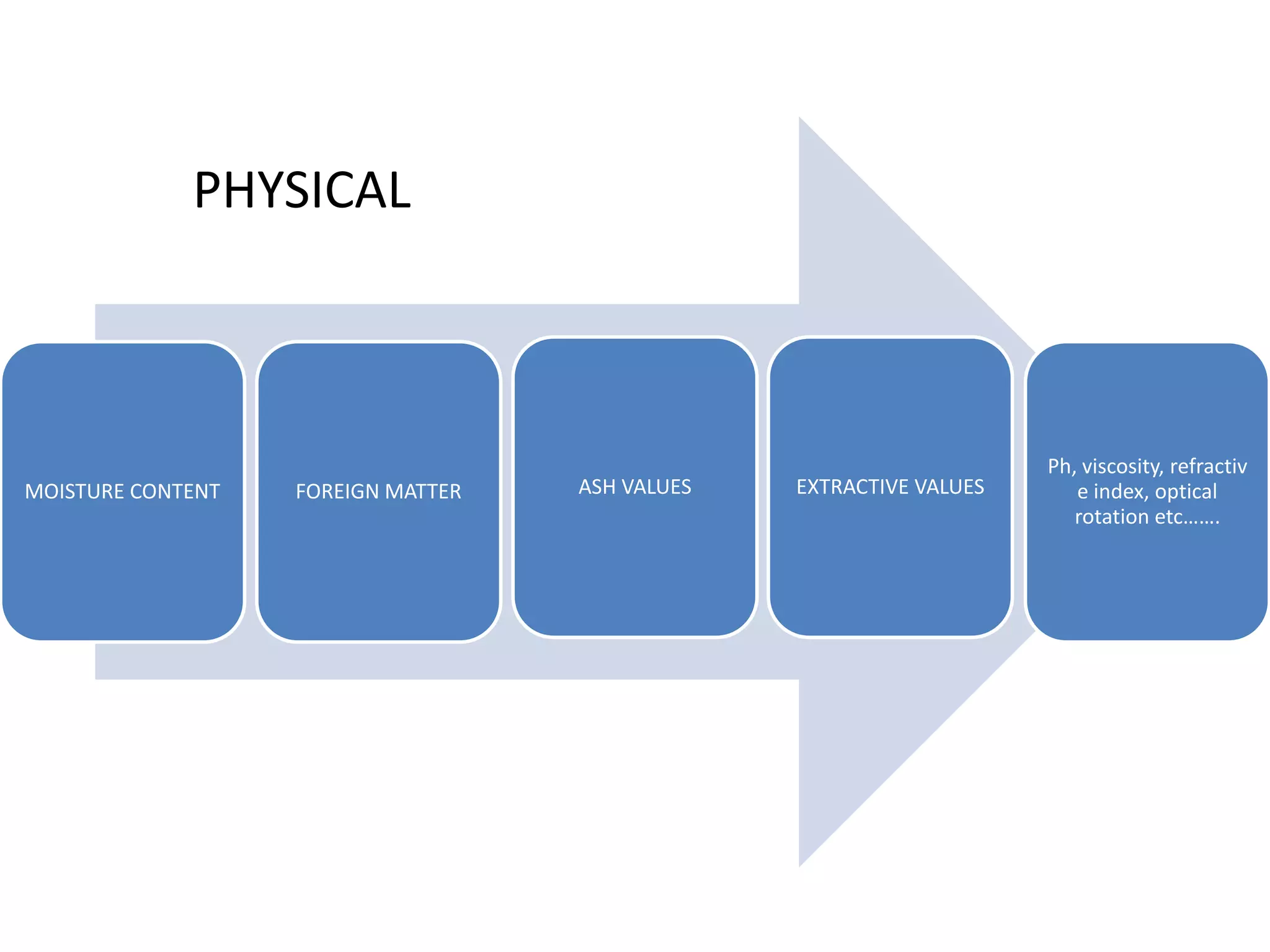
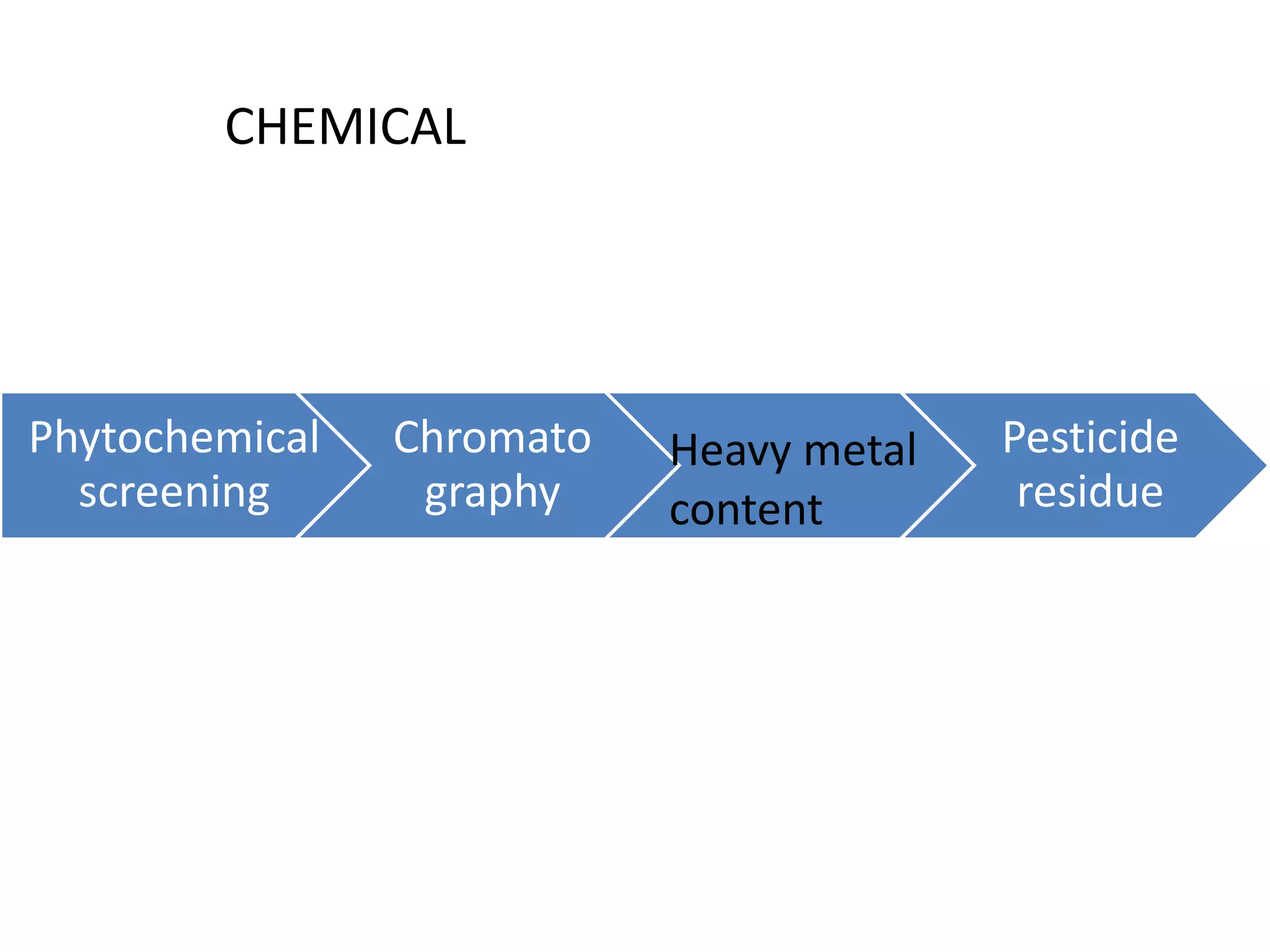
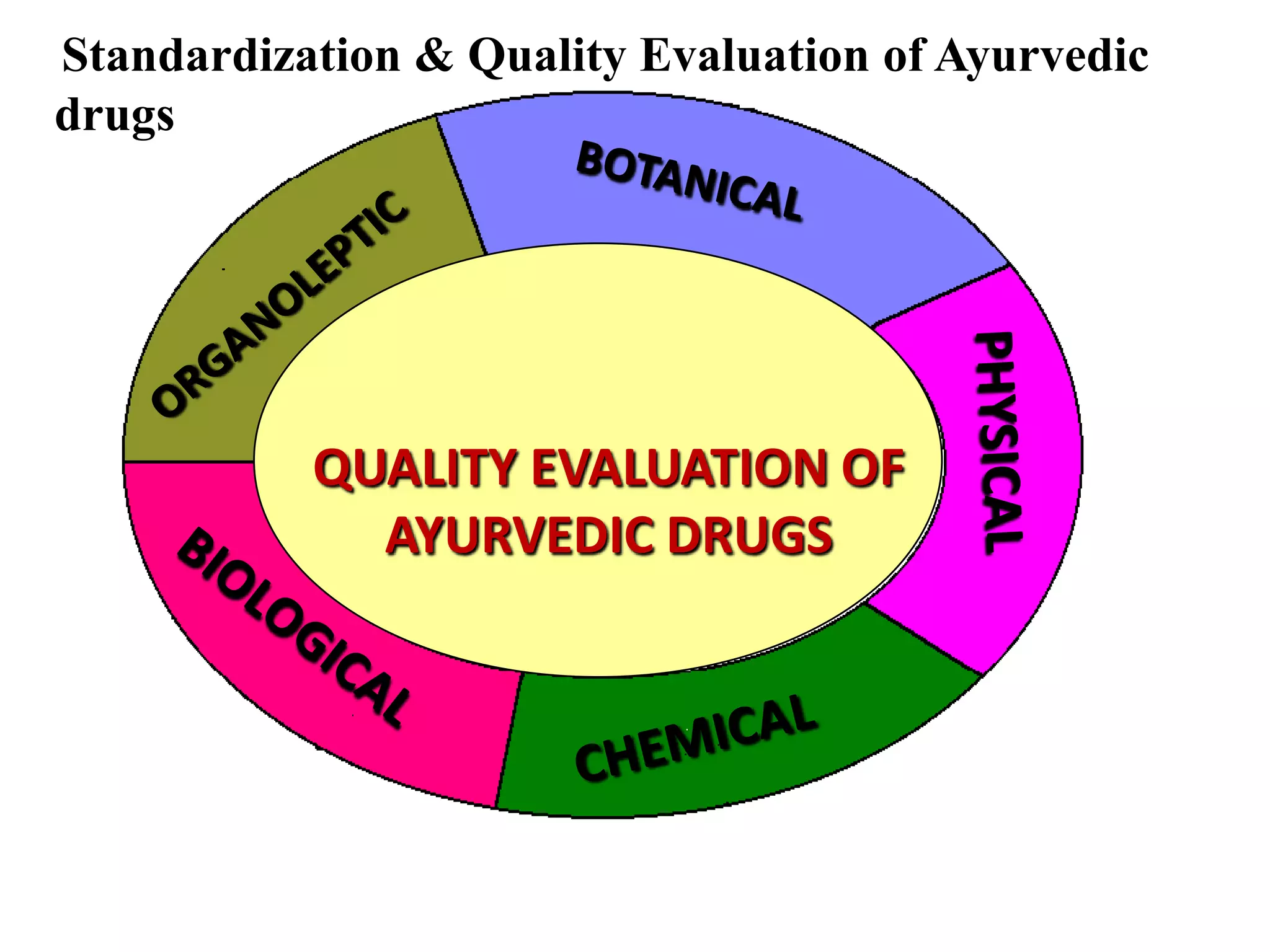
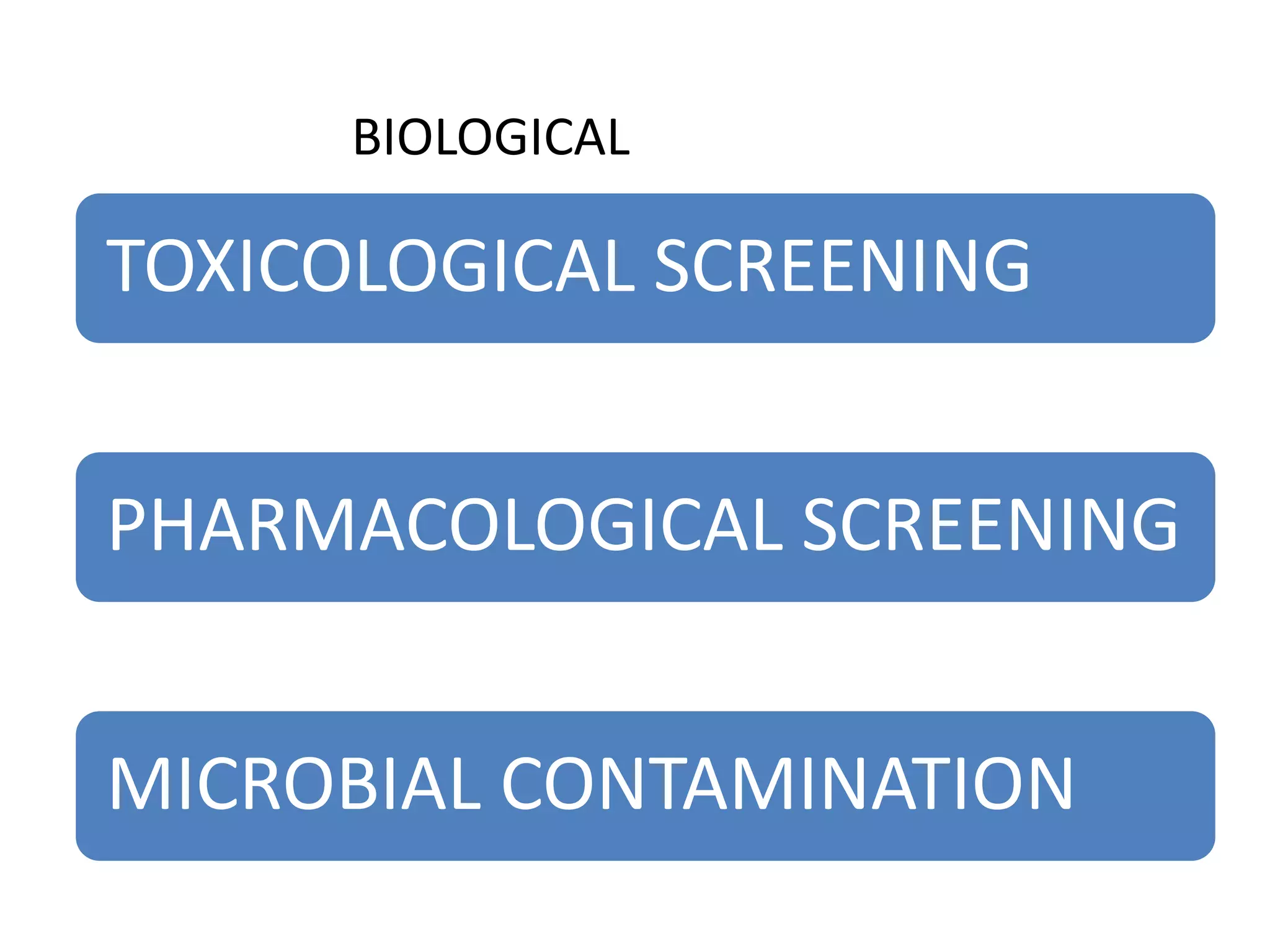
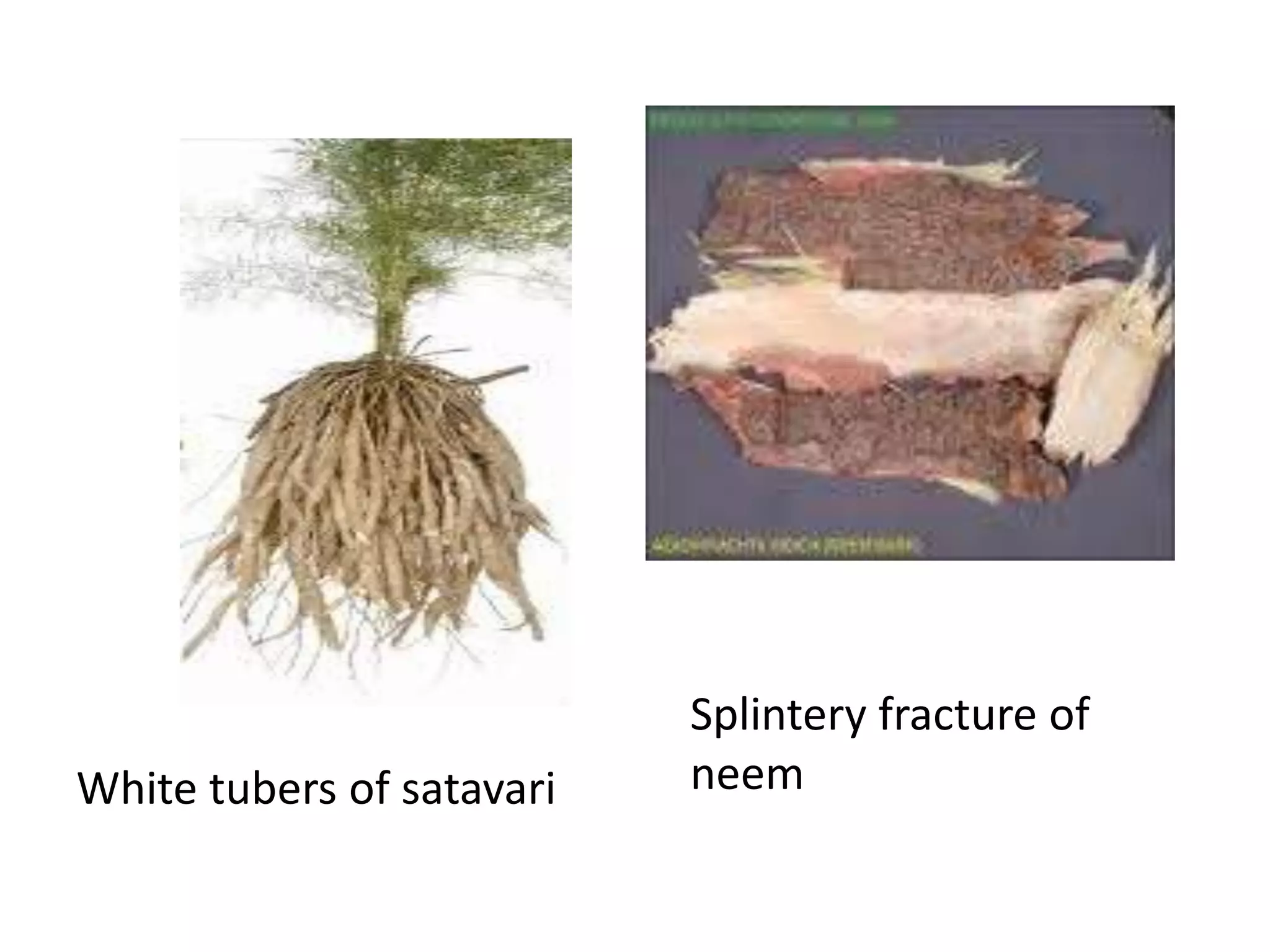
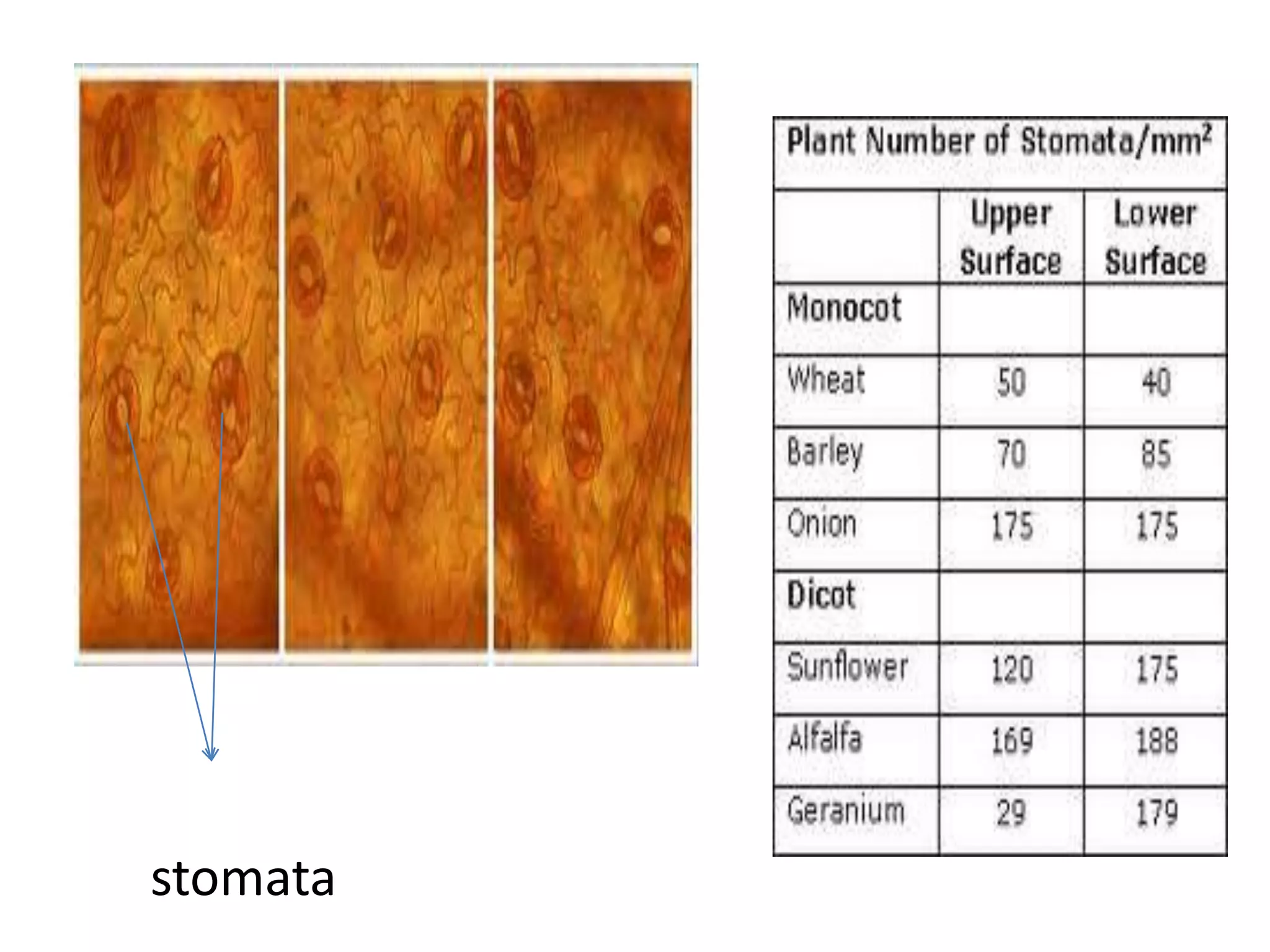
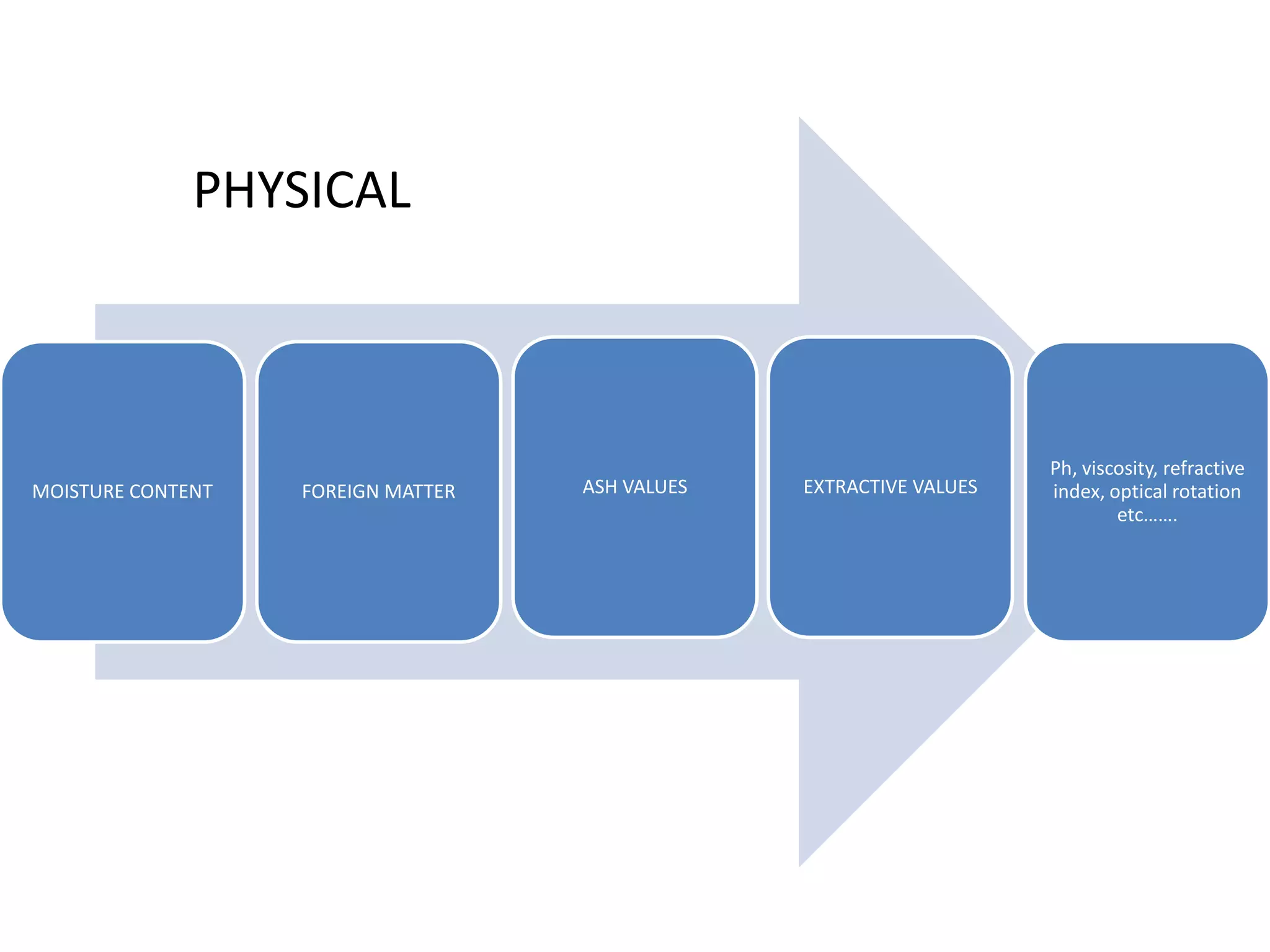
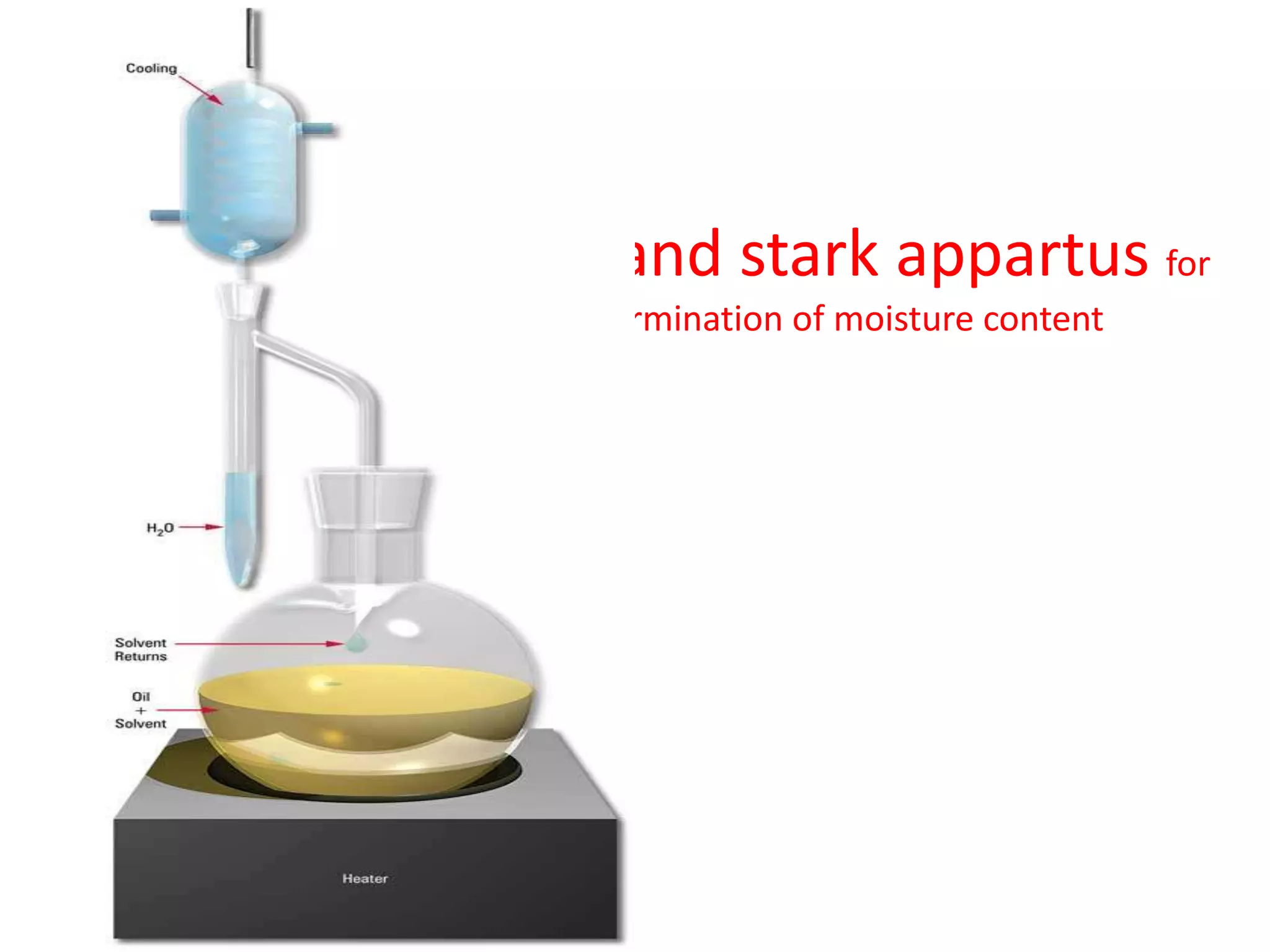
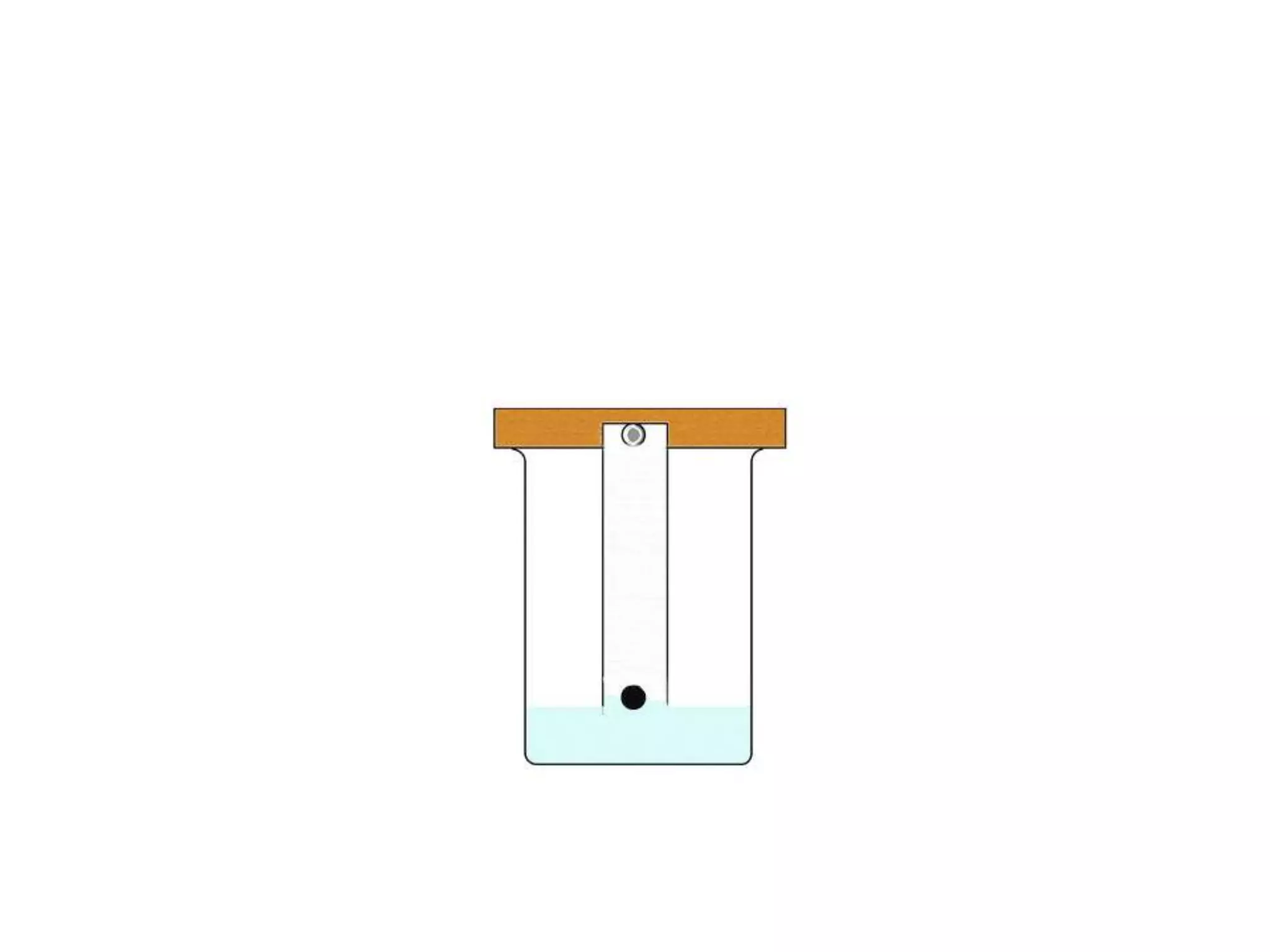
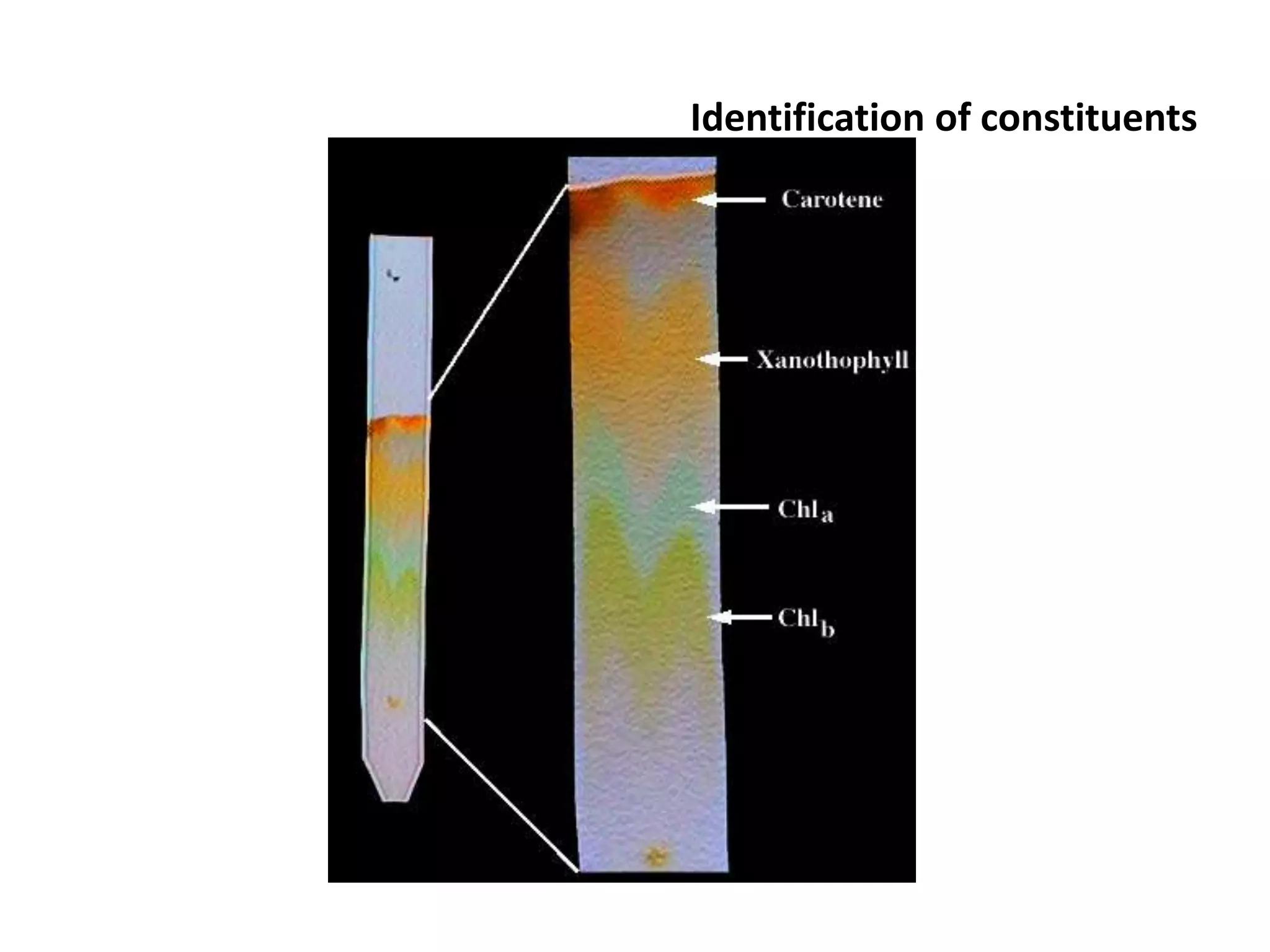
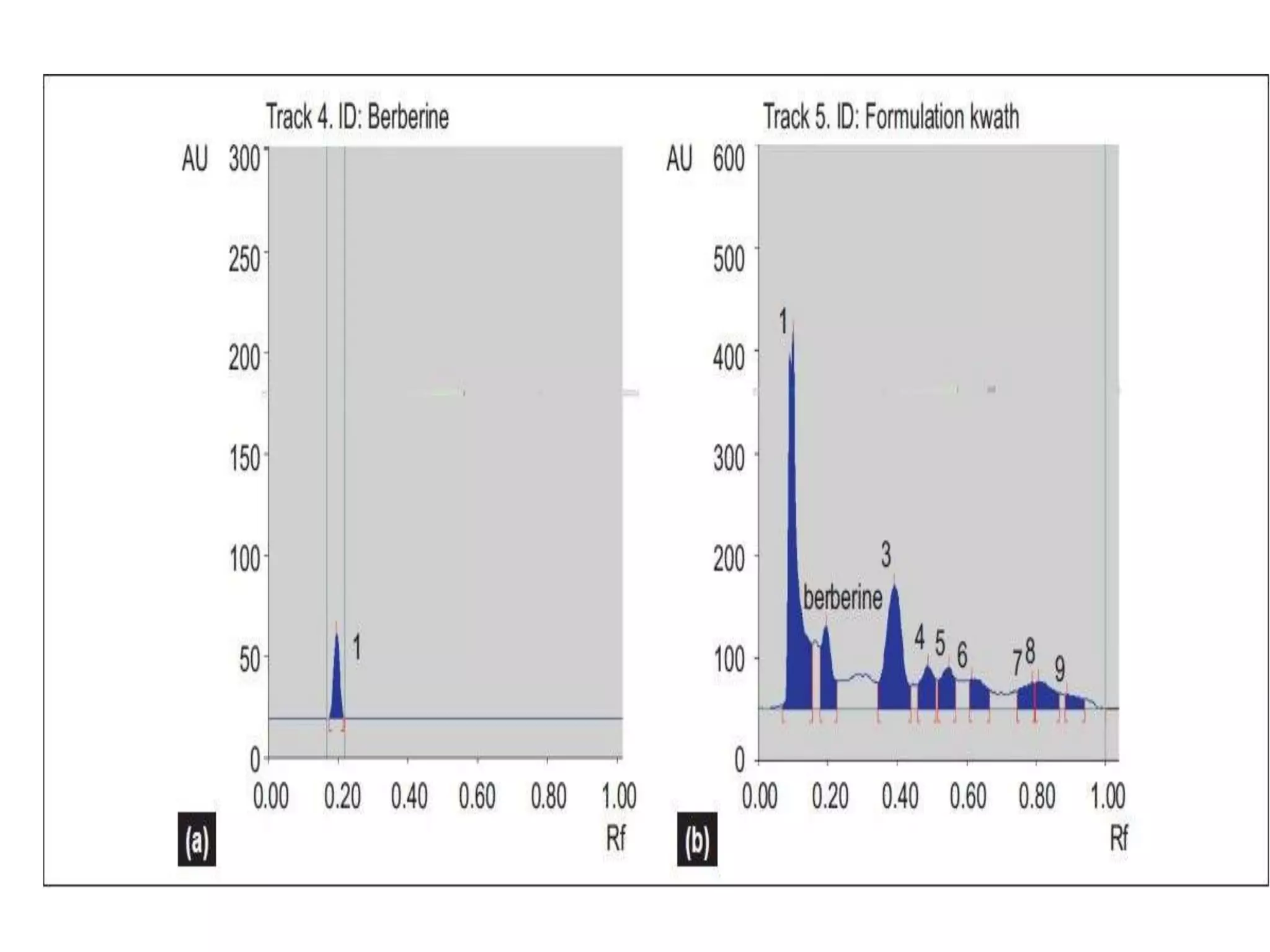
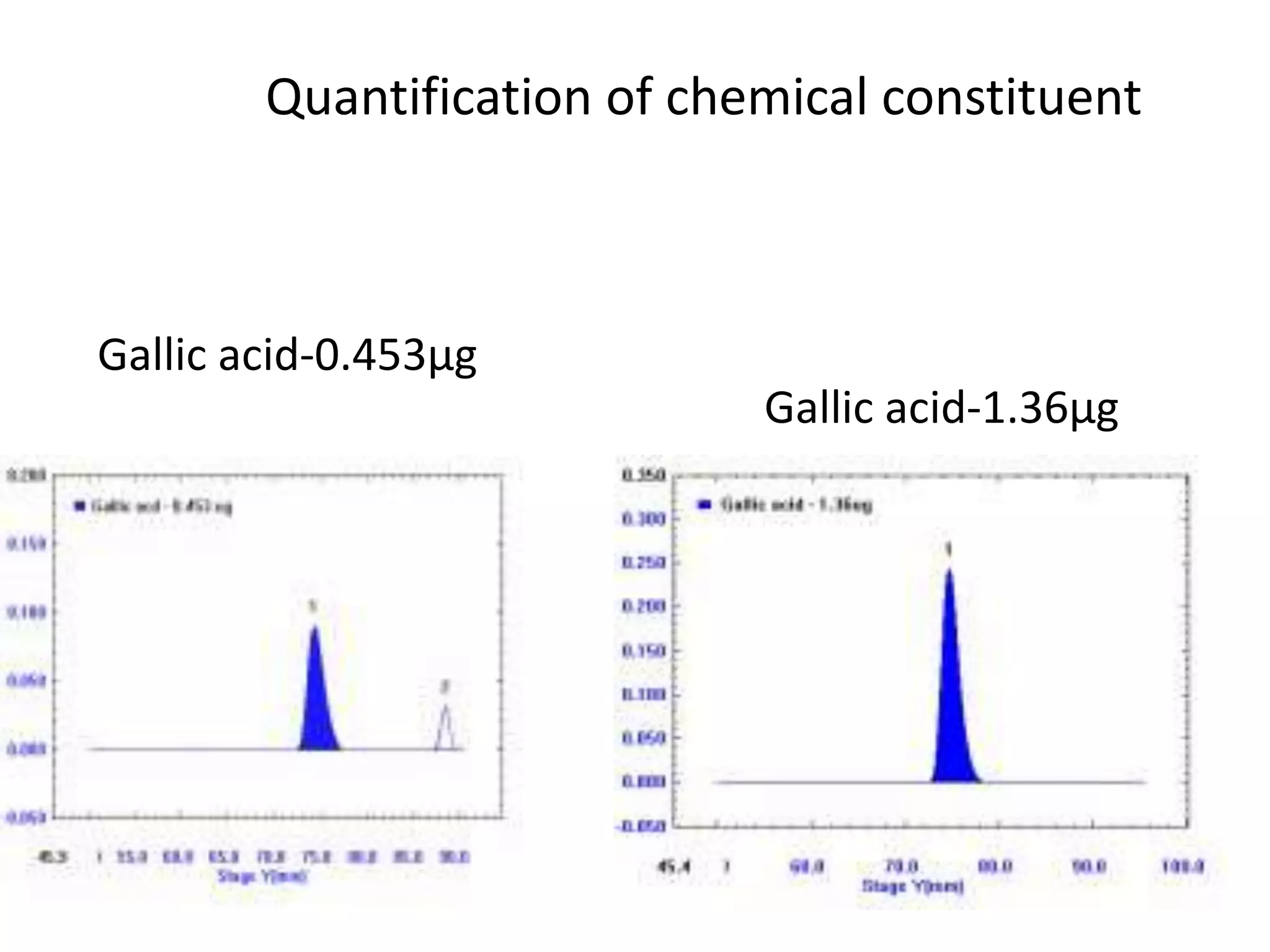
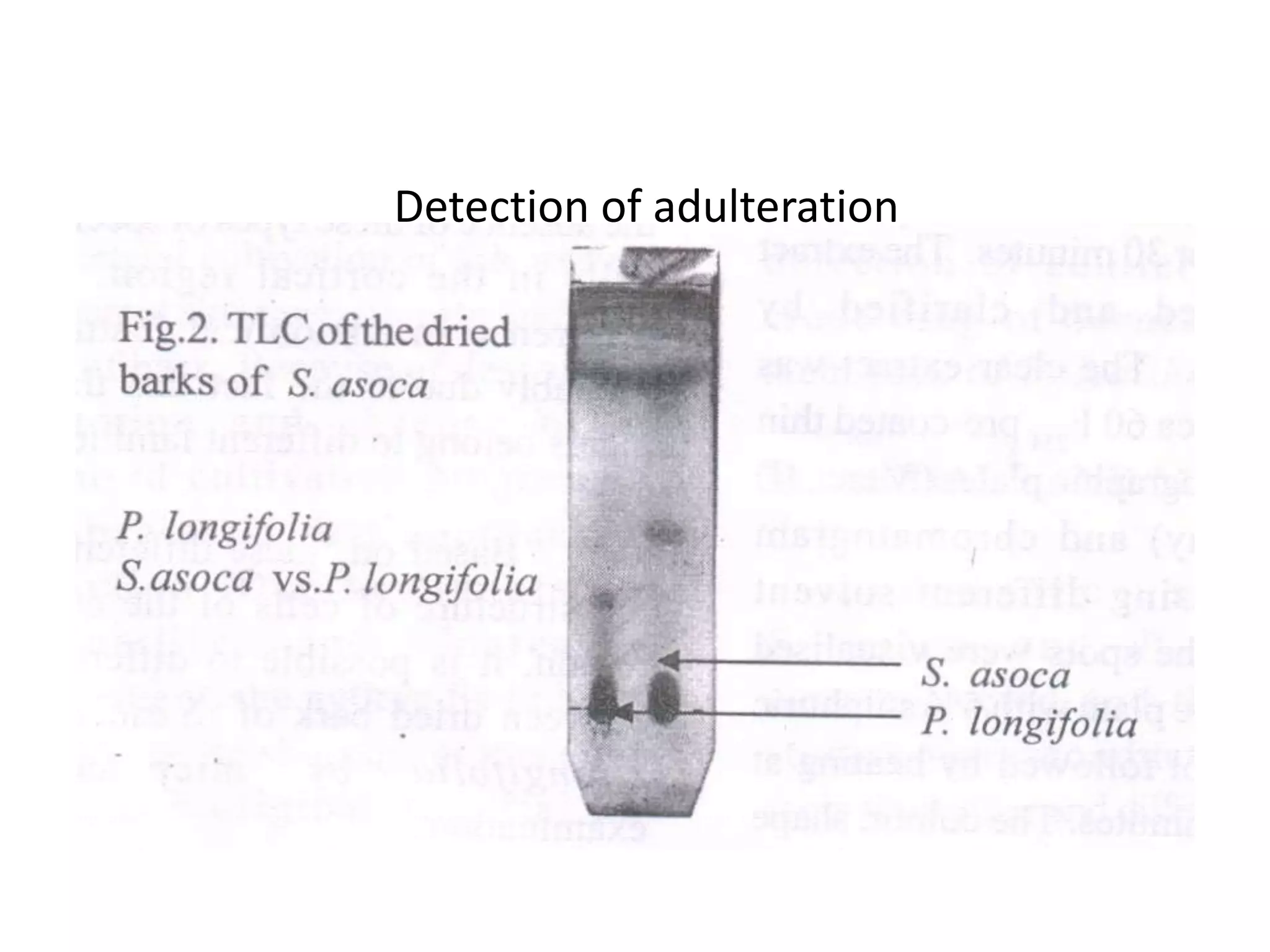
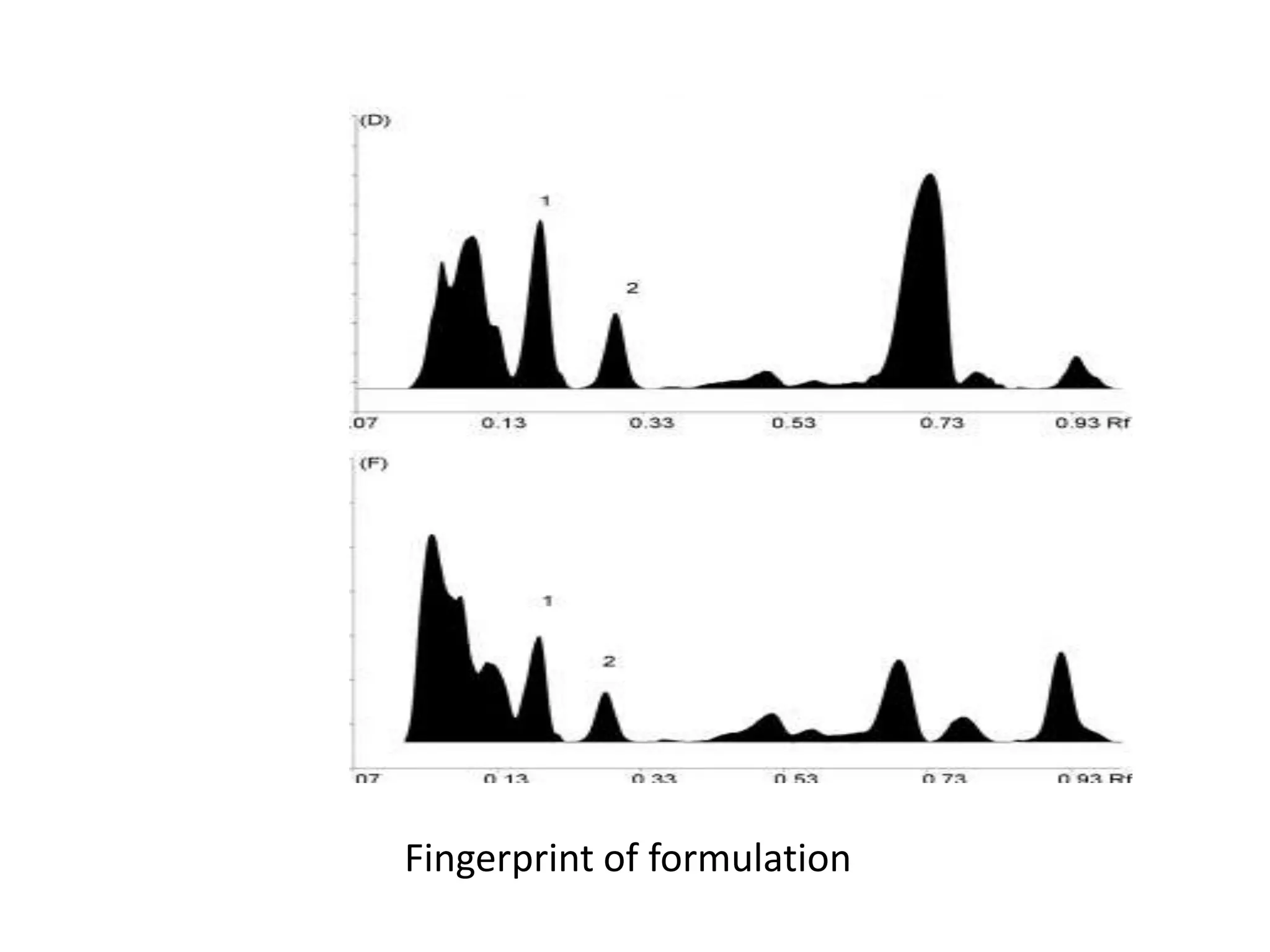
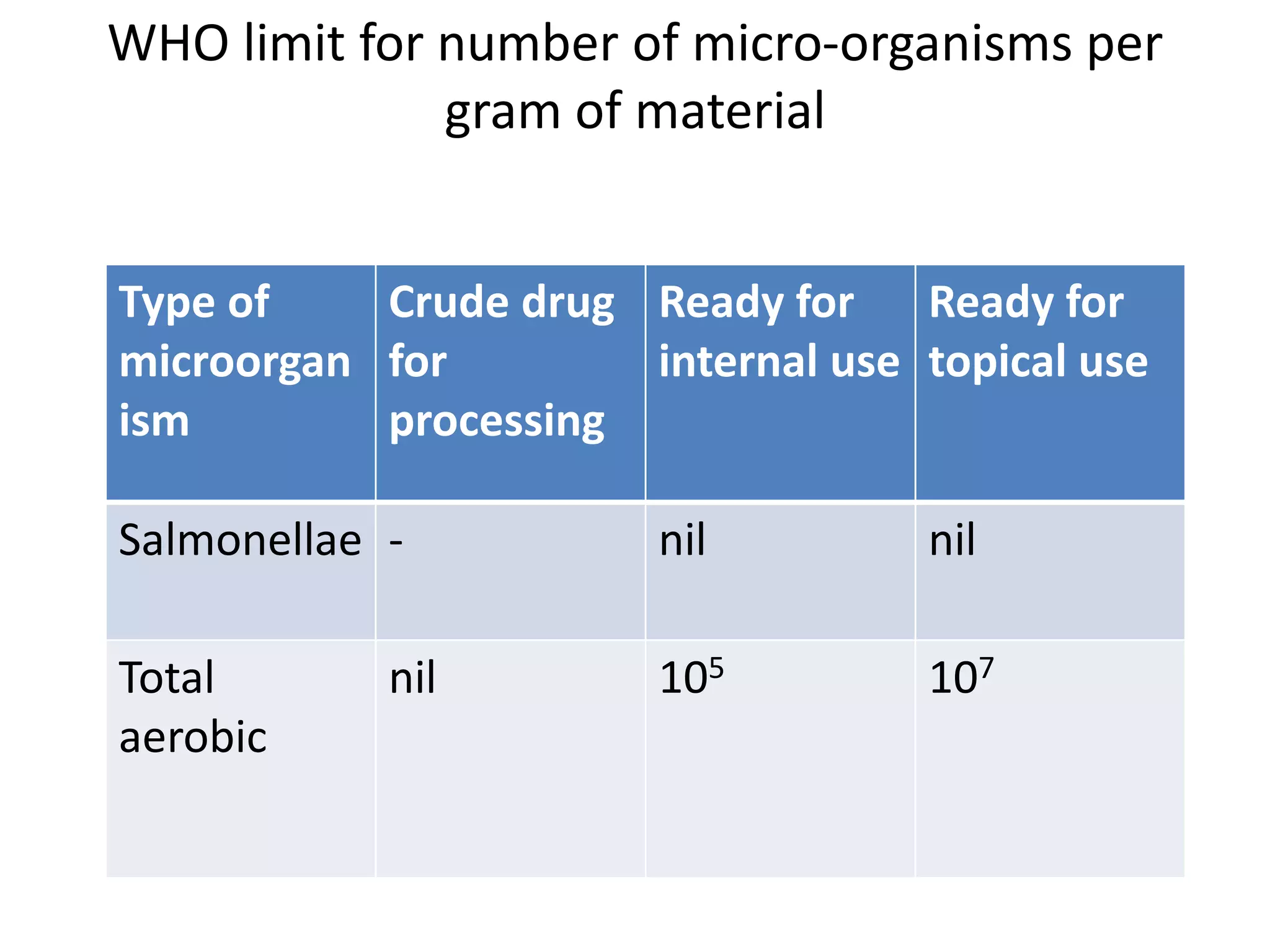
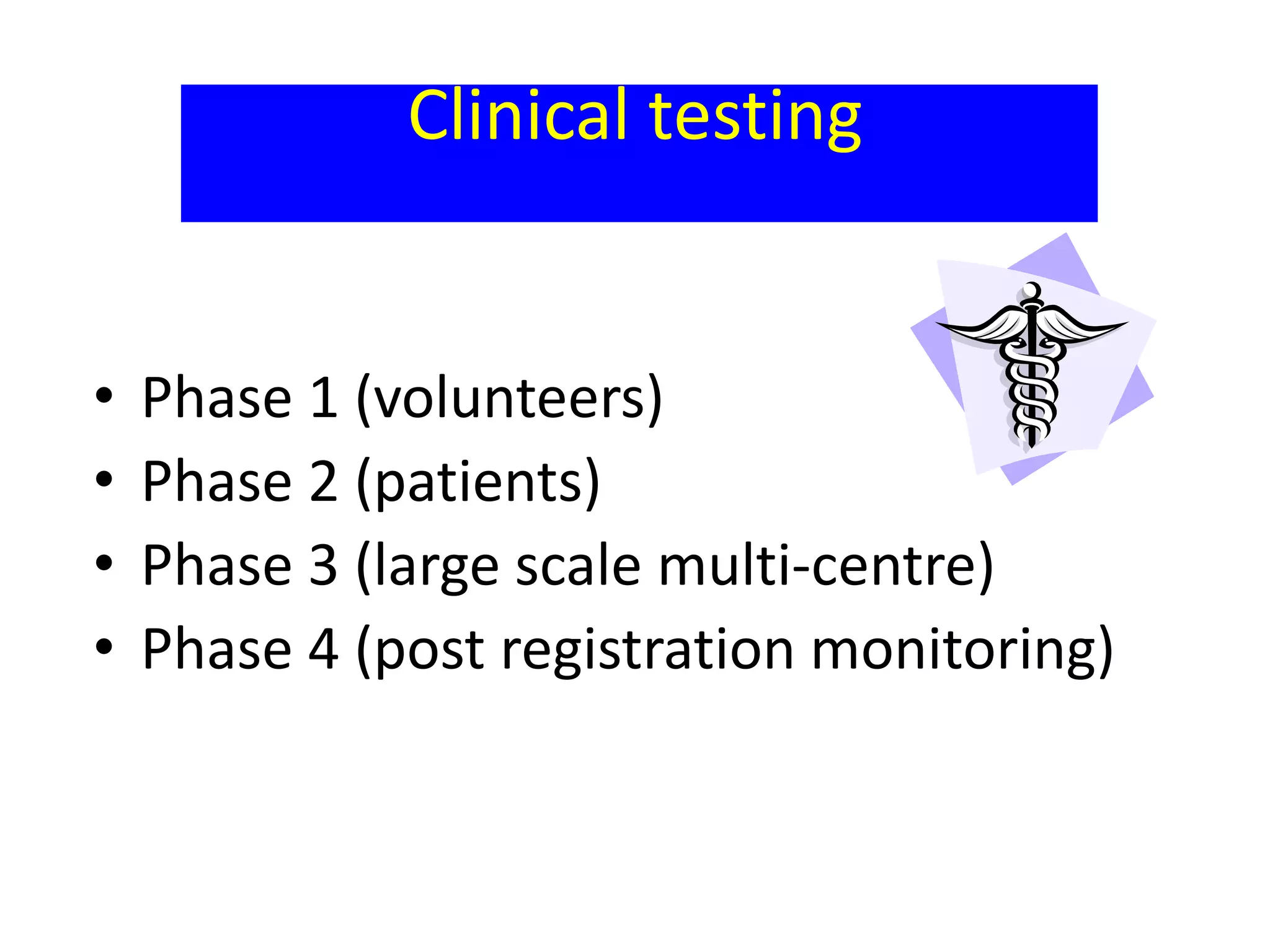
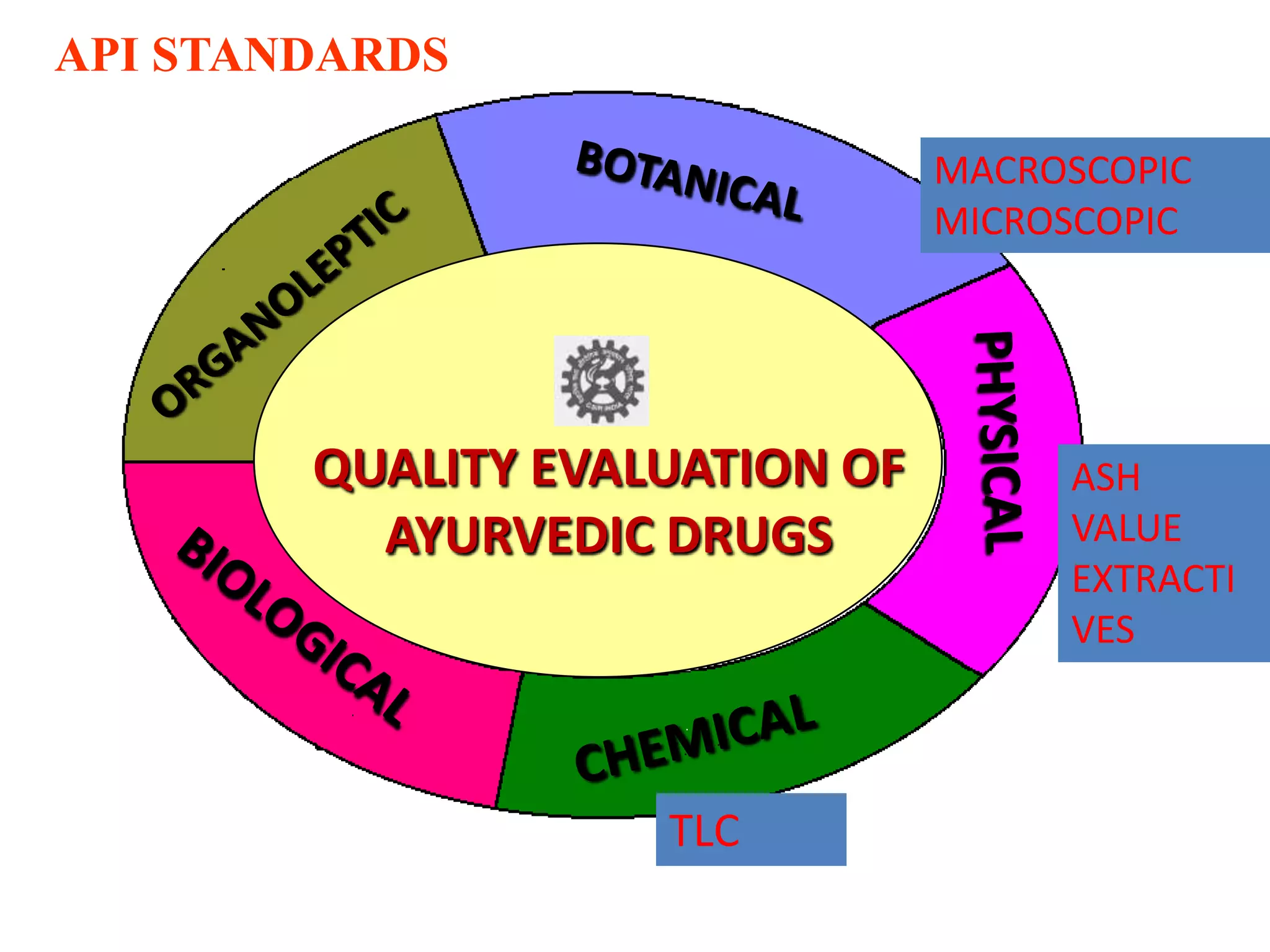
The document discusses quality control methods and standardization of Ayurvedic medicines, emphasizing the need for testing to identify drugs, detect adulteration, and establish reference standards. It outlines various evaluation techniques including organoleptic, physical, chemical, and biological assessments to ensure the purity and efficacy of Ayurvedic drugs. The text also covers the significance of pharmacological, toxicological, and microbial screenings in maintaining safety and quality in Ayurvedic practices.