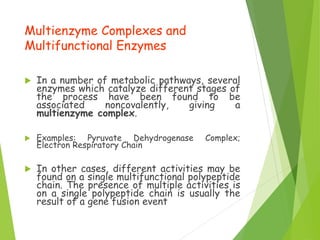
Enzymes are biomolecules that catalyze chemical reactions and lower their activation energy. They have high catalytic power, increasing reaction rates by factors of 108 to 1020. Enzymes also exhibit specificity, only catalyzing particular reactions and binding intended substrates. Regulation of enzyme activity ensures metabolic reactions proceed at appropriate rates. The International Union of Biochemistry and Molecular Biology established a systematic enzyme classification system based on reaction type. Enzymes are highly specific and can distinguish between stereoisomers, catalyze single reactions, and preferentially bind certain substrates. They exist as proteins, sometimes with tightly bound cofactors, and their tertiary structures are important for catalytic function.