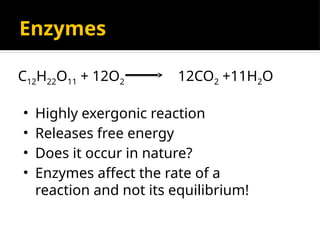
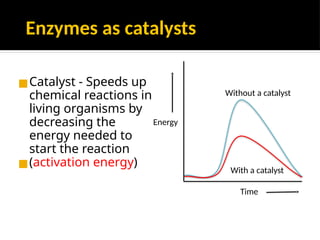
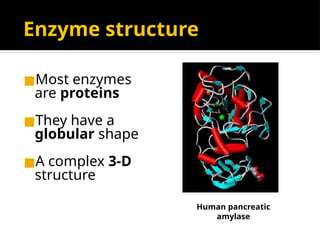
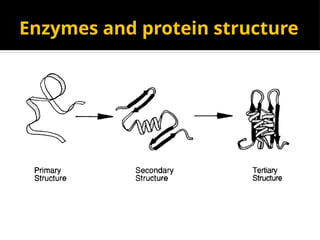
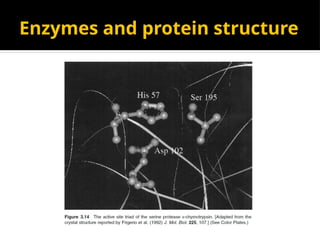
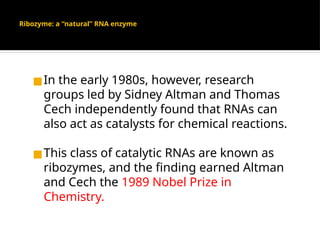
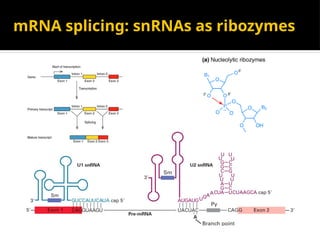
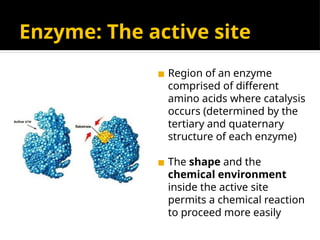
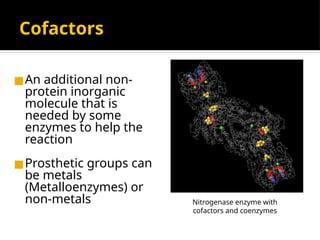
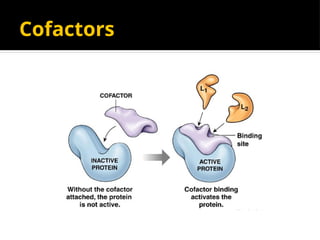
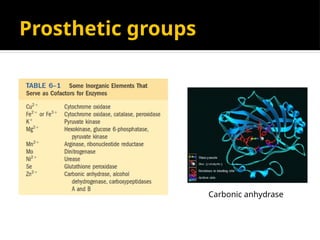
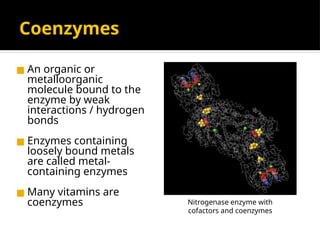
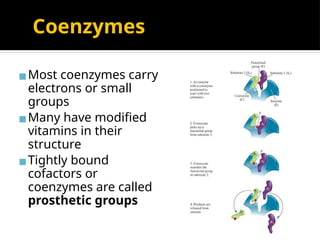
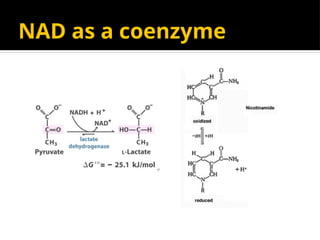
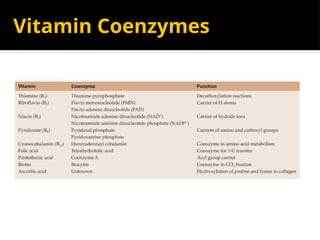
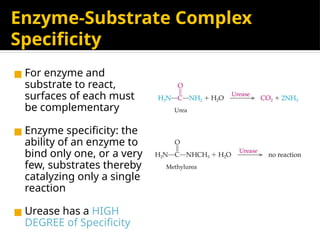
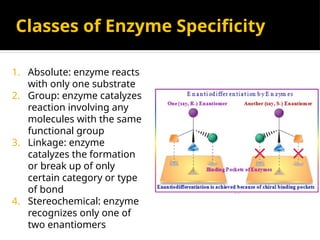
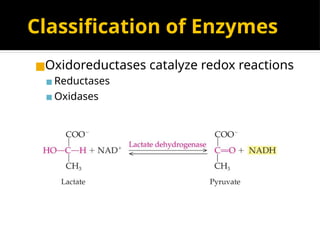
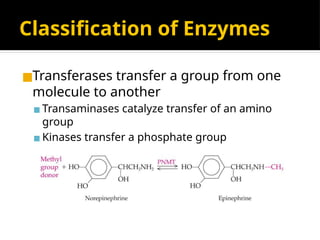
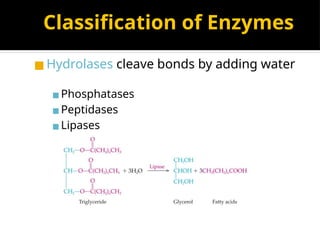
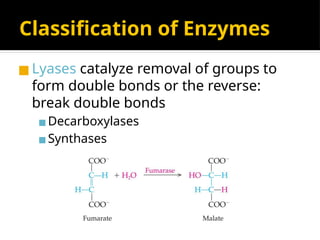
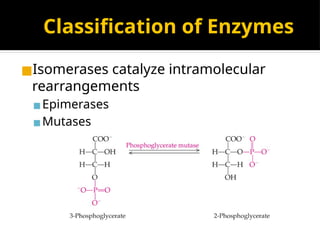
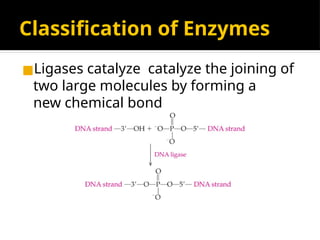
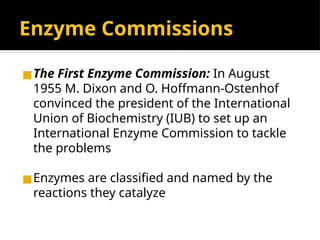
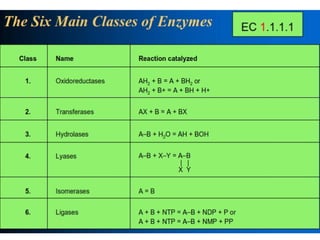
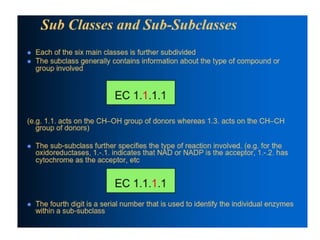
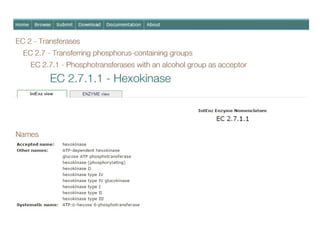
The document provides an overview of enzymes, their functions, structures, and classification in biological systems. Enzymes act as catalysts to speed up reactions, utilizing substrates and specific active sites while often requiring cofactors or coenzymes for activity. It also discusses enzyme nomenclature, the historical context for enzyme classification, and various types of enzymes based on their catalytic reactions.