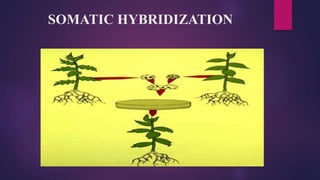
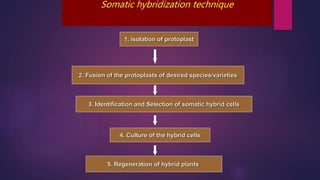
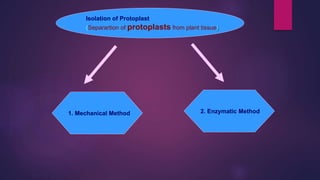
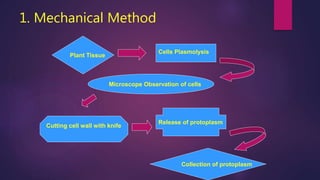
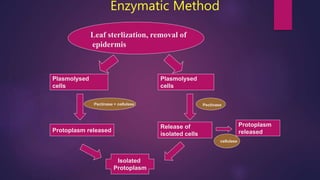
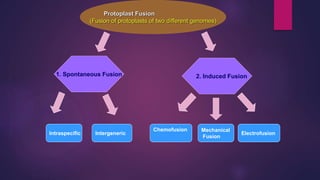
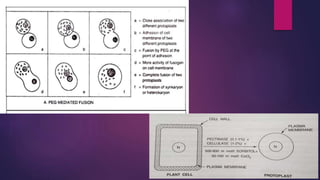
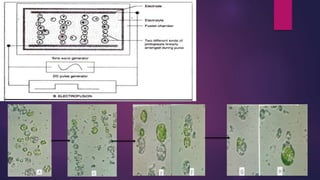
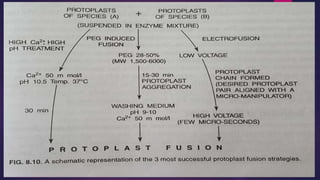
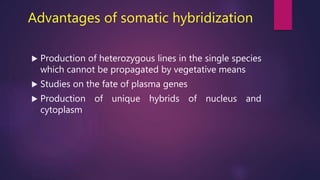
This document provides an introduction to somatic hybridization, which is the fusion of somatic protoplasts from two different plant species or varieties to create hybrid plants. It discusses the history and development of the technique, including early work isolating protoplasts. The key steps in somatic hybridization are described: isolating protoplasts using either mechanical or enzymatic methods, fusing the protoplasts spontaneously or through induction methods, identifying and selecting hybrid cells, culturing the hybrid cells, and regenerating hybrid plants. Advantages include creating novel hybrids and transferring desirable traits between species, while limitations include low regeneration rates and viability of fused products.