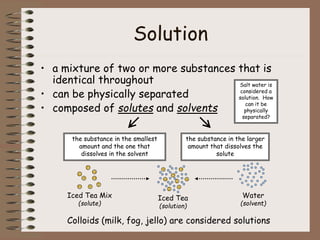
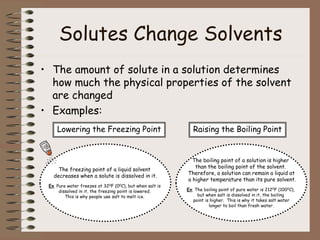
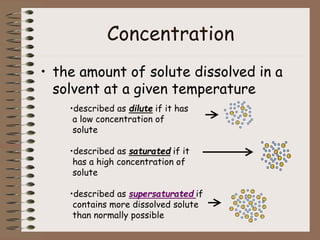
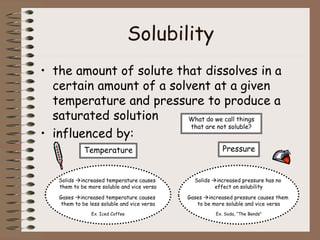
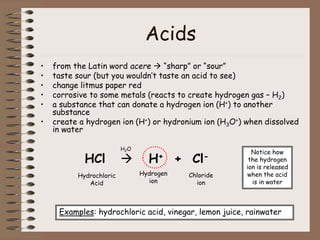
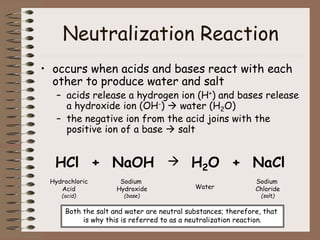
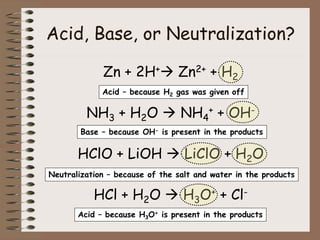
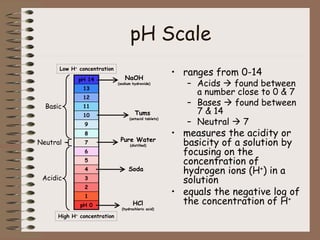
Mixtures can be either heterogeneous or homogeneous. Heterogeneous mixtures have visibly distinct components that can be separated physically, like a trail mix. Homogeneous mixtures appear uniform throughout and cannot be separated into distinct components using physical means, like salt water solutions. Solutions are a type of homogeneous mixture where a solute dissolves uniformly in a solvent. The concentration of a solute affects properties of the solvent like freezing point and boiling point. Acids donate hydrogen ions in water and turn litmus paper red, while bases accept hydrogen ions and turn litmus paper blue. A neutralization reaction occurs when an acid and base react and produce water and a salt. The pH scale measures acidity from 0-7 or basicity from