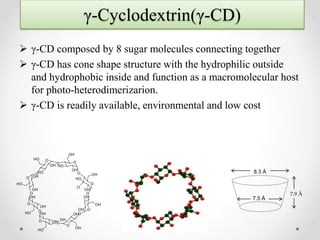
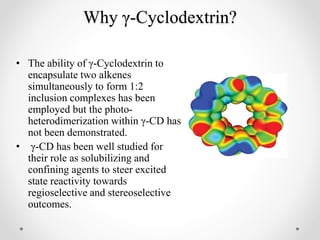
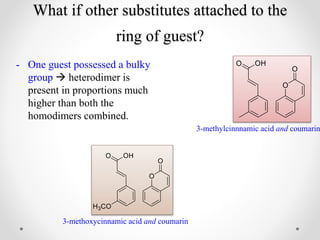
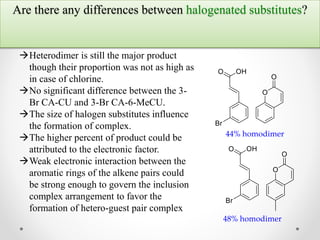
γ-Cyclodextrin can be used to control the orientation of alkenes and direct photodimerization reactions to form specific cyclobutane products. The study investigated the use of γ-cyclodextrin to direct the photodimerization of cinnamic acid and coumarin derivatives. Steric and electronic complementarity between the alkene guests governed the formation of hetero- versus homodimer complexes within the γ-cyclodextrin cavity. Substituents on the alkenes, like methyl and halogen groups, influenced the proportion of hetero- versus homodimers formed. The head-to-head heterodimer was generally the favored product when steric factors



















![References
Pattabiraman, M., & Clements, A. (2015). Journal of Photochemistry and
Photobiology A: Chemistry. γ-Cyclodextrin Mediated Photo-
heterodimerization between Cinnamic Acids and Coumarins, 297, 1-7.
Retrieved March 10, 2015, from
http://www.sciencedirect.com/science/article/pii/S1010603014004043
Pattabiraman, M., Natarajan, A., Kaanumalle, L., & Ramamurthy, V.
(2005). Organic Letter. Previous Article Next Article Table of Contents
Templating Photodimerization of Trans-Cinnamic Acids with Cucurbit[8]uril
and γ-Cyclodextrin, 7. Retrieved March 10, 2015, from
http://pubs.acs.org/doi/abs/10.1021/ol047866k
Maddipatla, M., Pattabiraman, M., Natarajan, A., Srivastav, K., Mague, J.,
& Ramamurthy, V. (2012). Organic & Biomolecular Chemistry.
Regioselective Photodimerization of Pyridyl-butadienes within
Curcubit[8]uril Cavities, 10(9219). Retrieved March 10, 2015, from
http://www.ncbi.nlm.nih.gov/pubmed/23103970](https://image.slidesharecdn.com/showcaseofficial-150316154621-conversion-gate01/85/Slide-Show-Presentation-26-320.jpg)