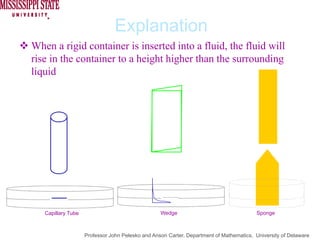
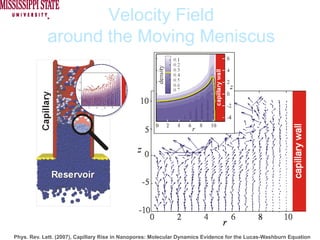
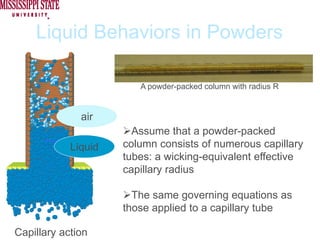
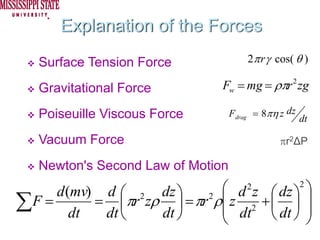
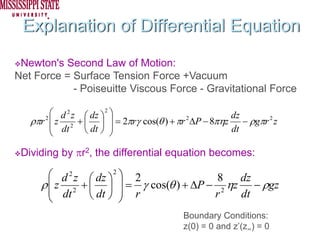
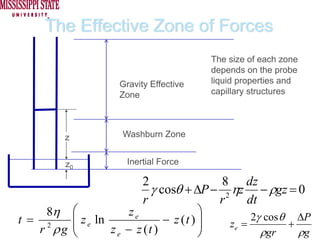
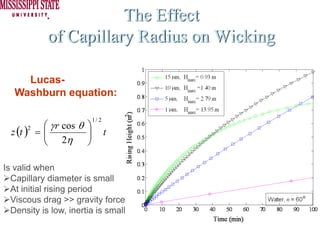
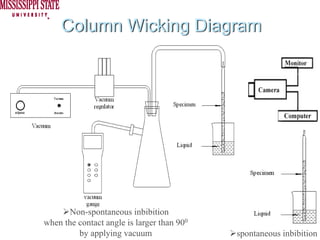
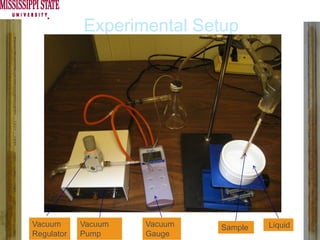
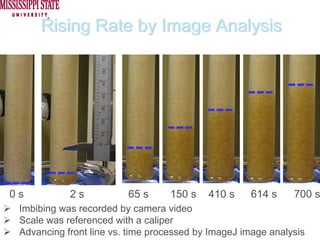
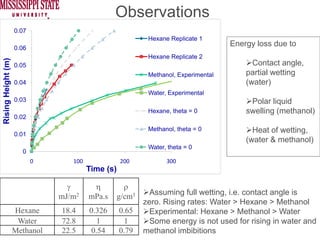
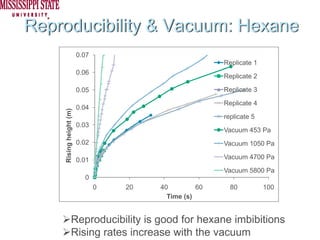
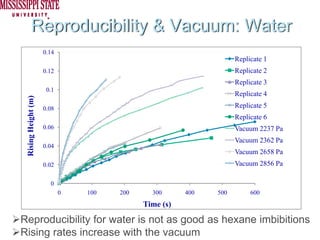
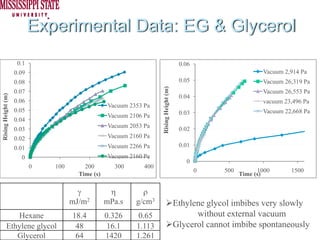
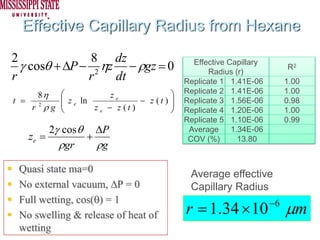
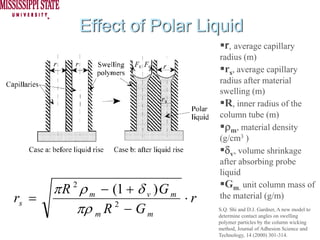
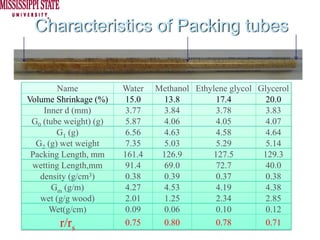
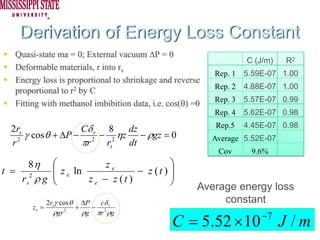
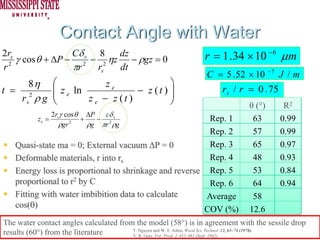
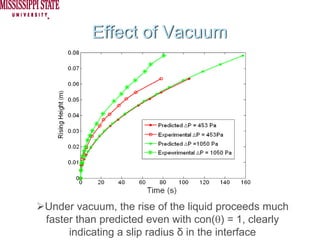
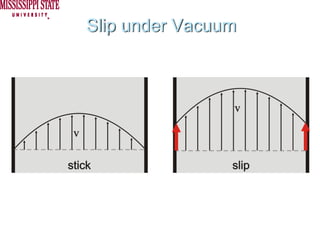
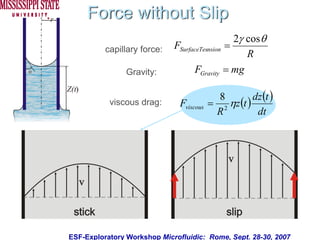
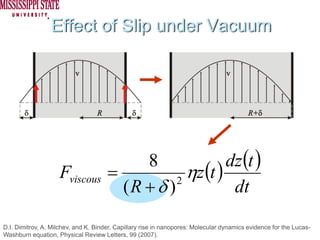
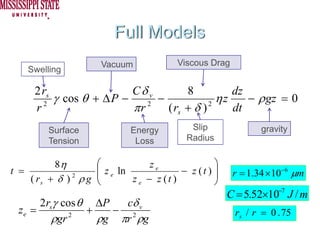
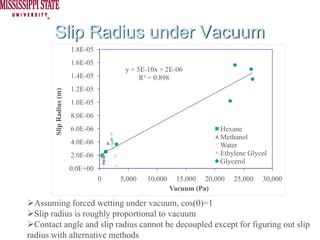
The document describes experiments on forced fluid imbibition in a powder-packed column. The objectives are to develop a tool to measure contact angles and surface energies for both spontaneous and non-spontaneous imbibing liquids in powders. The experiments apply vacuum to induce imbibition in cases where the wetting angle is larger than 90 degrees. Image analysis is used to measure rising rates of different liquids, including hexane, water, methanol, ethylene glycol and glycerol under varying vacuum conditions. The results show reproducibility is better for hexane than water, and rising rates increase with higher vacuum levels and lower liquid viscosity.