seran,,,slide share
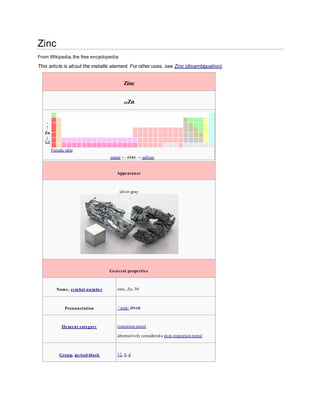
- 1. Zinc From Wikipedia, the free encyclopedia This article is about the metallic element. For other uses, see Zinc (disambiguation). Zinc 30Zn - ↑ Zn ↓ Cd Periodic table copper ← zinc → gallium Appearance silver-gray General properties Name, symbol ,number zinc, Zn, 30 Pronunciation /ˈzɪŋk/ ZINGK Element category t ransit ion metal alternat ively considered a post -t ransit ion metal Group, period,block 12, 4, d
- 2. Standard atomic weight 65.38(2) Electron configuration [Ar] 3d10 4s2 2, 8, 18, 2 History Discovery Indian metallurgists(before 1000 BC) First isolation Andreas Sigismund Marggraf(1746) Recognized as a unique metal by Rasaratna Samuccaya (800) Physical properties Phase solid Densi ty (nearr.t.) 7.14 g·cm−3 Liquid densi ty atm.p. 6.57 g·cm−3 Mel ting point 692.68 K, 419.53 °C, 787.15 °F Boi l ing point 1180 K, 907 °C, 1665 °F Heat of fusion 7.32 kJ·mol−1 Heat of vaporization 123.6 kJ·mol−1
- 3. Molar heat capaci ty 25.470 J·mol−1·K−1 Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 610 670 750 852 990 1179 Atomic properties Oxidation states +2, +1, 0 (amphoteric oxide) Electronegativi ty 1.65 (Pauling scale) Ionization energies (more) 1st : 906.4 kJ·mol−1 2nd: 1733.3 kJ·mol−1 3rd: 3833 kJ·mol−1 Atomic radius 134 pm Covalent radius 122±4 pm Van der Waals radius 139 pm Miscel lanea Crystal structure hexagonal close-packed Magnetic ordering diamagnet ic Electrical resistivi ty (20 °C) 59.0 nΩ·m
- 4. Thermal conductivi ty 116 W·m−1·K−1 Thermal expansion (25 °C) 30.2 μm·m−1·K−1 Speed of sound(thin rod) (r.t .) (rolled) 3850 m·s−1 Young's modulus 108 GPa Shear modulus 43 GPa Bulk modulus 70 GPa Poisson ratio 0.25 Mohs hardness 2.5 Brinel l hardness 412 MPa CAS registry number 7440-66-6 Most stable isotopes Main art icle: Isotopes of zinc iso NA hal f-l i fe DM DE (MeV) DP 64Zn 48.6% >2.3×1018 y β+β+ 1.096 64Ni 65Zn syn 243.8 d ε 1.3519 65Cu γ 1.1155 - 66Zn 27.9% 66Zn is stable with 36 neut rons 67Zn 4.1% 67Zn is stable with 37 neut rons 68Zn 18.8% 68Zn is stable with 38 neut rons 69Zn syn 56 min β− 0.906 69Ga 69mZn syn 13.76 h β− 0.906 69Ga 70Zn 0.6% >1.3×1016 y β−β− 0.998 70Ge 71Zn syn 2.4 min β− 2.82 71Ga 71mZn syn 3.97 d β− 2.82 71Ga 72Zn syn 46.5 h β− 0.458 72Ga
- 5. V T E · r Zinc, in commerce also spelter, is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element of group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2. Zinc is the 24th most abundant element in the Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest mineable amounts are found in Australia, Asia, and the United States. Zinc production includes froth flotation of the ore, roasting, and final extraction using electricity (electrowinning). Brass, which is an alloy of copper and zinc, has been used since at least the 10th century BC. Impure zinc metal was not produced in large scale until the 13th century in India, while the metal was unknown to Europe until the end of the 16th century. Alchemists burned zinc in air to form what they called "philosopher's wool" or "white snow." The element was probably named by the alchemist Paracelsus after the German word Zinke. German chemist Andreas Sigismund Marggraf is normally given credit for discovering pure metallic zinc in 1746. Work by Luigi Galvani and Alessandro Volta uncovered the electrochemical properties of zinc by 1800. Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Other applications are inbatteries, small non-structural castings, and alloys, such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate andzinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory. Zinc is an essential mineral of "exceptional biologic and public health importance".[1] Zinc deficiency affects about two billion people in the developing world and is associated with many diseases. [2] In children it causes growth retardation, delayed sexual maturation, infection susceptibility, and diarrhea, contributing to the death of about 800,000 children worldwide per year.[1] Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as alcohol dehydrogenase in humans. Consumption of excess zinc can cause ataxia, lethargy andcopper deficiency. Contents [hide]
- 6. 1 Characteristics o 1.1 Physical properties o 1.2 Occurrence o 1.3 Isotopes 2 Compounds and chemistry o 2.1 Reactivity o 2.2 Zinc(I) compounds o 2.3 Zinc (II) compounds 3 History o 3.1 Ancient use o 3.2 Early studies and naming o 3.3 Isolation o 3.4 Later work 4 Production o 4.1 Mining and processing o 4.2 Environmental impact 5 Applications o 5.1 Anti-corrosion and batteries o 5.2 Alloys o 5.3 Other industrial uses o 5.4 Dietary supplement o 5.5 Topical use o 5.6 Organic chemistry 6 Biological role o 6.1 Enzymes o 6.2 Other proteins o 6.3 Dietary intake o 6.4 Deficiency o 6.5 Dreaming 6.5.1 Argriculture 7 Precautions o 7.1 Toxicity
- 7. o 7.2 Poisoning 8 See also 9 Notes 10 References 11 Bibliography 12 External links Characteristics Physical properties Zinc, also referred to in nonscientific contexts as spelter,[3] is a bluish-white, lustrous, diamagnetic metal,[4] though most common commercial grades of the metal have a dull finish. [5] It is somewhat less dense than iron and has a hexagonal crystal structure.[6] The metal is hard and brittle at most temperatures but becomes malleable between 100 and 150 °C.[4][5] Above 210 °C, the metal becomes brittle again and can be pulverized by beating. [7] Zinc is a fair conductor of electricity.[4] For a metal, zinc has relatively low melting (419.5 °C, 787.1 F) and boiling points (907 °C).[8] Its melting point is the lowest of all the transition metals aside from mercury and cadmium.[8] Many alloys contain zinc, including brass, an alloy of copper and zinc. Other metals long known to form binary alloys with zinc are aluminium,antimony, bismuth, gold, iron, lead, mercury, silver, tin, magnesium, cobalt, nickel, tellurium and sodium.[9] While neither zinc nor zirconium areferromagnetic, their alloy ZrZn2 exhibits ferromagnetism below 35 K.[4] Occurrence See also: Zinc minerals Zinc makes up about 75 ppm (0.0075%) of the Earth's crust, making it the 24th most abundant element. Soil contains 5–770 ppm of zinc with an average of 64 ppm. Seawater has only 30 ppb zinc and the atmosphere contains 0.1–4 μg/m3.[10]
- 8. Sphalerite (ZnS) The element is normally found in association with other base metals such as copper and lead in ores.[11] Zinc is achalcophile, meaning the element has a low affinity for oxides and prefers to bond with sulfides. Chalcophiles formed as the crust solidified under the reducing conditions of the early Earth's atmosphere.[12] Sphalerite, which is a form of zinc sulfide, is the most heavily mined zinc-containing ore because its concentrate contains 60–62% zinc.[11] Other minerals from which zinc is extracted include smithsonite (zinc carbonate), hemimorphite (zinc silicate),wurtzite (another zinc sulfide), and sometimes hydrozincite (basic zinc carbonate).[13] With the exception of wurtzite, all these other minerals were formed as a result of weathering processes on the primordial zinc sulfides.[12] Identified world zinc resources total about 1.9 billion tonnes.[14] Large deposits are in Australia, Canada and the United States with the largest reserves in Iran.[12][15][16] At the current rate of consumption, these reserves are estimated to be depleted sometime between 2027 and 2055. [17][18] About 346 million tonnes have been extracted throughout history to 2002, and one estimate found that about 109 million tonnes of that remains in use.[19] Isotopes Main article: Isotopes of zinc Five isotopes of zinc occur in nature. 64Zn is the most abundant isotope (48.63% natural abundance).[20] This isotope has such a long half-life, at4.3×1018 a,[21] that its radioactivity can be ignored.[22] Similarly, 70Zn (0.6%), with a half-life of 1.3×1016 a is not usually considered to be radioactive. The other isotopes found in nature are 66Zn (28%), 67Zn (4%) and 68Zn (19%). Several dozen radioisotopes have been characterized. 65Zn, which has a half-life of 243.66 days, is the most long-lived radioisotope, followed by 72Znwith a half-life of 46.5 hours.[20] Zinc has 10 nuclear isomers. 69mZn has the longest half-life, 13.76 h.[20] The superscript m indicates a metastableisotope. The nucleus of a metastable isotope is in an excited state and will return to the ground state by emitting a photon in the form of a gamma ray. 61Zn has three excited states and 73Zn has two.[23] The isotopes 65Zn, 71Zn, 77Zn and 78Zn each have only one excited state.[20] The most common decay mode of a radioisotope of zinc with a mass number lower than 66 is electron capture. The decay product resulting from electron capture is an isotope of copper.[20] n 30Zn + e− → n 29Cu
- 9. The most common decay mode of a radioisotope of zinc with mass number higher than 66 is beta decay (β–), which produces an isotope ofgallium.[20] n 30Zn → n 31Ga + e− + ν e Compounds and chemistry Main article: Compounds of zinc Reactivity Zinc has an electron configuration of [Ar]3d104s2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent.[24] The surface of the pure metal tarnishes quickly, eventually forming a protective passivating layer of the basic zinc carbonate, Zn5(OH)6(CO3)2, by reaction with atmospheric carbon dioxide.[25] This layer helps prevent further reaction with air and water. Zinc burns in air with a bright bluish-green flame, giving off fumes of zinc oxide.[26] Zinc reacts readily with acids, alkalis and other non-metals.[27] Extremely pure zinc reacts only slowly at room temperature with acids.[26] Strong acids, such as hydrochloric or sulfuric acid, can remove the passivating layer and subsequent reaction with water releases hydrogen gas. [26] The chemistry of zinc is dominated by the +2 oxidation state. When compounds in this oxidation state are formed the outer shell s electrons are lost, which yields a bare zinc ion with the electronic configuration [Ar]3d10.[28] In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species.[29] The volatilization of zinc in combination with zinc chloride at temperatures above 285 °C indicates the formation of Zn2Cl2, a zinc compound with a +1 oxidation state.[26] No compounds of zinc in oxidation states other than +1 or +2 are known. [30]Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist. [31] Zinc chemistry is similar to the chemistry of the late first -row transition metals nickel and copper, though it has a filled d-shell, so its compounds are diamagnetic and mostly colorless.[32] Theionic radii of zinc and magnesium happen to be nearly identical. Because of this some of their salts have the same crystal structure[33] and in circumstances where ionic radius is a determining factor zinc and magnesium chemistries have much in common.[26] Otherwise there is little similarity. Zinc tends to form bonds with a greater degree of covalency and it forms much more stablecomplexes with N-and S- donors.[32] Complexes of zinc are mostly 4- or 6- coordinate although 5-coordinate complexes are known.[26]
- 10. See also Clemmensen reduction. Zinc(I) compounds Zinc(I) compounds are rare, and requires bulky ligands to stabilize the low oxidation state. Most zinc(I) compounds contains formally the [Zn2]2+ core, which is analogous to the [Hg2]2+ dimeric cation present in mercury(I) compounds. The diamagnetic nature of the ion confirms its dimeric structure. The first zinc(I) compound containing the Zn—Zn bond, (η5-C5Me5)2Zn2, is also the firstdimetallocene. The [Zn2]2+ ion rapidly disproportionates into zinc metal and zinc(II), and has only been obtained as a yellow glass formed by cooling a solution of metallic zinc in molten ZnCl2.[34] Zinc (II) compounds Zinc acetate Zinc chloride Binary compounds of zinc are known for most of the metalloids and all the nonmetals except the noble gases. The oxide ZnOis a white powder that is nearly insoluble in neutral aqueous solutions, but is amphoteric, dissolving in both strong basic and acidic solutions.[26] The other chalcogenides (ZnS, ZnSe, and ZnTe) have varied applications in electronics and optics.[35]Pnictogenides (Zn3N2, Zn3P2, Zn3As2 and Zn3Sb2),[36][37] the peroxide (ZnO2), the hydride (ZnH2), and the carbide (ZnC2) are also known.[38] Of the four halides, ZnF2 has the most ionic character, whereas the others (ZnCl2, ZnBr2, and ZnI2) have relatively low melting points and are considered to have more covalent character.[39] In weak basic solutions containing Zn2+ ions, the hydroxide Zn(OH)2 forms as a white precipitate. In stronger alkaline solutions, this hydroxide is dissolved to form zincates ([Zn(OH)4]2−).[26] The
- 11. nitrate Zn(NO3)2, chlorate Zn(ClO3)2, sulfateZnSO4, phosphate Zn3(PO4)2, molybdate ZnMoO4, cyanide Zn(CN)2, arsenite Zn(AsO2)2, arsenate Zn(AsO4)2·8H2O and the chromate ZnCrO4 (one of the few colored zinc compounds) are a few examples of other common inorganic compounds of zinc.[40][41] One of the simplest examples of an organic compound of zinc is the acetate (Zn(O2CCH3)2). Organozinc compounds are those that contain zinc–carbon covalent bonds. Diethylzinc ((C2H5)2Zn) is a reagent in synthetic chemistry. It was first reported in 1848 from the reaction of zinc andethyl iodide, and was the first compound known to contain a metal–carbon sigma bond.[42] History Ancient use Late Roman brass bucket – theHemmoorer Eimer f rom Warstade, Germany, second to third century AD Various isolated examples of the use of impure zinc in ancient times have been discovered. A possibly prehistoric statuette containing 87.5% zinc was found in a Dacian archaeological site in Transylvania (modern Romania).[43] Ornaments made of alloys that contain 80–90% zinc with lead, iron, antimony, and other metals making up the remainder, have been found that are 2500 years old.[11] The Berne zinc tablet is a votive plaque dating to Roman Gaul made of an alloy that is mostly zinc.[44] Also, some ancient writings appear to mention zinc. The Greek historian Strabo, in a passage taken from an earlier writer of the 4th century BC, mentions "drops of false silver", which when mixed with copper make brass. This may refer to small quantities of zinc by-product of smelting sulfideores.[45] The Charaka Samhita, thought to have been written in 500 BC or before, mentions a metal which, when oxidized, produces pushpanjan, thought to be zinc oxide.[46] Zinc ores were used to make the zinc–copper alloy brass many centuries prior to the discovery of zinc as a separate element. Judean brass from the 14th to 10th centuries BC contains 23% zinc.[47] The Book of Genesis, written between the 10th and 5th centuries BC, [48] mentions (in the King James
- 12. translation)Tubal-cain as an "instructor of every artificer in brass and iron" (Genesis 4:22), but since the word nechosheth, translated as "brass", also means "copper", the significance of this is not clear. Knowledge of how to produce brass spread to Ancient Greece by the 7th century BC but few varieties were made.[49] The manufacture of brass was known to the Romans by about 30 BC.[50] They made brass by heating powdered calamine (zinc silicate or carbonate), charcoal and copper together in a crucible. [50] The resulting calamine brass was then either cast or hammered into shape and was used in weaponry.[51] Some coins struck by Romans in the Christian era are made of what is probably calamine brass.[52] In the West, impure zinc was known from antiquity to exist in the remnants in melting ovens, but it was usually discarded, as it was thought to be worthless. [53] Zinc mines at Zawar, near Udaipur in India, have been active since the Mauryan period in the late 1st millennium BC. The smelting of metallic zinc here however appears to have begun around the 12th century AD.[54][55] One estimate is that this location produced an estimated million tonnes of metallic zinc and zinc oxide from the 12th to 16th centuries. [13] Another estimate gives a total production of 60,000 tonnes of metallic zinc over this period. [54] The Rasaratna Samuccaya, written in approximately the 13th century AD, mentions two types of zinc-containing ores; one used for metal extraction and another used for medicinal purposes.[55] Early studies and naming Zinc was distinctly recognized as a metal under the designation of Yasada or Jasada in the medical Lexicon ascribed to the Hindu king Madanapala and written about the year 1374.[56] Smelting and extraction of impure zinc by reducing calamine with wool and other organic substances was accomplished in the 13th century in India.[4][57] The Chinese did not learn of the technique until the 17th century.[57] Various alchemical symbols attributed to the element zinc Alchemists burned zinc metal in air and collected the resulting zinc oxide on a condenser. Some alchemists called this zinc oxide lana philosophica, Latin for "philosopher's wool", because it collected in wooly tufts while others thought it looked like white snow and named it nix album.[58] The name of the metal was probably first documented by Paracelsus, a Swiss-born German alchemist, who referred to the metal as "zincum" or "zinken" in his book Liber Mineralium II, in the 16th
- 13. century.[57][59] The word is probably derived from the German zinke, and supposedly meant "tooth-like, pointed or jagged" (metallic zinc crystals have a needle-like appearance).[60] Zink could also imply "tin-like" because of its relation to German zinnmeaning tin.[61] Yet another possibility is that the word is derived from the Persian word س نگ seng meaning stone.[62] The metal was also called Indian tin, tutanego, calamine, and spinter.[11] German metallurgist Andreas Libavius received a quantity of what he called "calay" of Malabar from a cargo ship captured from the Portuguese in 1596. [63] Libavius described the properties of the sample, which may have been zinc. Zinc was regularly imported to Europe from the Orient in the 17th and early 18th centuries,[57] but was at times very expensive.[note 1] Isolation Andreas Sigismund Marggraf is given credit for f irst isolating pure zinc The isolation of metallic zinc in the West may have been achieved independently by several people. Postlewayt's Universal Dictionary, a contemporary source giving technological information in Europe, did not mention zinc before 1751 but the element was studied before then. [55][64] Flemish metallurgist P.M. de Respour reported that he extracted metallic zinc from zinc oxide in 1668.[13] By the start of the 18th century, Étienne François Geoffroy described how zinc oxide condenses as yellow crystals on bars of iron placed above zinc ore being smelted. [13] In Britain, John Lane is said to have carried out experiments to smelt zinc, probably at Landore, prior to his bankruptcy in 1726.[65] In 1738, William Champion patented in Great Britain a process to extract zinc from calamine in a vertical retort style smelter.[66] His technology was somewhat similar to that used at Zawar zinc mines in Rajasthan but there is no evidence that he visited the Orient.[67] Champion's process was used through 1851.[57]
- 14. German chemist Andreas Marggraf normally gets credit for discovering pure metallic zinc even though Swedish chemist Anton von Swab distilled zinc from calamine four years before.[57] In his 1746 experiment, Marggraf heated a mixture of calamine and charcoal in a closed vessel without copper to obtain a metal.[53] This procedure became commercially practical by 1752. [68] Later work Galvanization w as named af terLuigi Galvani. William Champion's brother, John, patented a process in 1758 for calcining zinc sulfide into an oxide usable in the retort process.[11] Prior to this only calamine could be used to produce zinc. In 1798, Johann Christian Ruberg improved on the smelting process by building the first horizontal retort smelter.[69] Jean-Jacques Daniel Dony built a different kind of horizontal zinc smelter in Belgium, which processed even more zinc.[57] Italian doctor Luigi Galvani discovered in 1780 that connecting the spinal cord of a freshly dissected frog to an iron rail attached by a brass hook caused the frog's leg to twitch.[70] He incorrectly thought he had discovered an ability of nerves and muscles to create electricity and called the effect "animal electricity".[71] The galvanic cell and the process of galvanization were both named for Luigi Galvani and these discoveries paved the way for electrical batteries, galvanization and cathodic protection.[71] Galvani's friend, Alessandro Volta, continued researching this effect and invented the Voltaic pile in 1800.[70] The basic unit of Volta's pile was a simplifiedgalvanic cell, which is made of a plate of copper and a plate of zinc connected to each other externally and separated by an electrolyte. These were stacked in series to make the Voltaic cell, which in turn produced electricity by directing electrons from the zinc to the copper and allowing the zinc to corrode. [70] The non-magnetic character of zinc and its lack of color in solution delayed discovery of its importance to biochemistry and nutrition.[72] This changed in 1940 when carbonic anhydrase, an enzyme that
- 15. scrubs carbon dioxide from blood, was shown to have zinc in its active site.[72] The digestive enzymecarboxypeptidase became the second known zinc-containing enzyme in 1955.[72] Production Mining and processing Top zinc output countries 2010[14] Rank Country Tonnes 1 China 3,500,000 2 Peru 1,520,000 3 Australia 1,450,000 4 India 750,000 5 United States 720,000 6 Canada 670,000 Main articles: Zinc mining and Zinc smelting See also: List of countries by zinc production Percentage of zinc output in 2006 by countries[73]
- 16. Zinc is the fourth most common metal in use, trailing only iron, aluminium, and copper with an annual production of about 12 million tonnes.[14] The world's largest zinc producer is Nyrstar, a merger of the Australian OZ Minerals and the Belgian Umicore.[74] About 70% of the world's zinc originates from mining, while the remaining 30% comes from recycling secondary zinc.[75] Commercially pure zinc is known as Special High Grade, often abbreviated SHG, and is 99.995% pure.[76] Worldwide, 95% of the zinc is mined from sulfidic ore deposits, in which sphalerite ZnS is nearly always mixed with the sulfides of copper, lead and iron. [77]There are zinc mines throughout the world, with the main mining areas being China, Australia and Peru. China produced 29% of the global zinc output in 2010.[14] Zinc metal is produced using extractive metallurgy.[78] After grinding the ore, froth flotation, which selectively separates minerals from gangue by taking advantage of differences in their hydrophobicity, is used to get an ore concentrate.[78] A final concentration of zinc of about 50% is reached by this process with the remainder of the concentrate being sulfur (32%), iron (13%), and SiO2(5%).[78] Roasting converts the zinc sulfide concentrate produced during processing to zinc oxide:[77] 2 ZnS + 3 O2 → 2 ZnO + 2 SO2 The sulfur dioxide is used for the production of sulfuric acid, which is necessary for the leaching process. If deposits of zinc carbonate, zinc silicate or zinc spinel, like the Skorpion Deposit in Namibia are used for zinc production the roasting can be omitted. [79] For further processing two basic methods are used: pyrometallurgy or electrowinning. Pyrometallurgy processing reduces zinc oxide with carbon or carbon monoxide at 950 °C (1,740 °F) into the metal, which is distilled as zinc vapor. [80] The zinc vapor is collected in a condenser.[77] The below set of equations demonstrate this process: [77] 2 ZnO + C → 2 Zn + CO2 ZnO + CO → Zn + CO2 Electrowinning processing leaches zinc from the ore concentrate by sulfuric acid:[81] ZnO + H2SO4 → ZnSO4 + H2O After this step electrolysis is used to produce zinc metal.[77] 2 ZnSO4 + 2 H2O → 2 Zn + 2 H2SO4 + O2 The sulfuric acid regenerated is recycled to the leaching step. Environmental impact
- 17. The production for sulfidic zinc ores produces large amounts of sulfur dioxide and cadmium vapor. Smelter slag and other residues of process also contain significant amounts of heavy metals. About 1.1 million tonnes of metallic zinc and 130 thousand tonnes of lead were mined and smelted in the Belgian towns of La Calamine and Plombières between 1806 and 1882.[82] The dumps of the past mining operations leach significant amounts of zinc and cadmium, and, as a result, the sediments of the Geul River contain significant amounts of heavy metals.[82] About two thousand years ago emissions of zinc from mining and smelting totaled 10 thousand tonnes a year. After increasing 10-fold from 1850, zinc emissions peaked at 3.4 million tonnes per year in the 1980s and declined to 2.7 million tonnes in the 1990s, although a 2005 study of the Arctic troposphere found that the concentrations there did not reflect the decline. Anthropogenic and natural emissions occur at a ratio of 20 to 1.[83] Levels of zinc in rivers flowing through industrial or mining areas can be as high as 20 ppm.[84] Effective sewage treatment greatly reduces this; treatment along the Rhine, for example, has decreased zinc levels to 50 ppb.[84] Concentrations of zinc as low as 2 ppm adversely affects the amount of oxygen that fish can carry in their blood.[85] Historically responsible for high heavy metal levels in the Derw ent River,[86] the zinc w orks at Lutana is the largest exporter in Tasmania, generating 2.5% of the state's GDP, and producing over 250 thousand tonnes of zinc per year.[87] Soils contaminated with zinc through the mining of zinc-containing ores, refining, or where zinc-containing sludge is used as fertilizer, can contain several grams of zinc per kilogram of dry soil. Levels of zinc in excess of 500 ppm in soil interfere with the ability of plants to absorb other essential metals, such as iron and manganese. Zinc levels of 2000 ppm to 180,000 ppm (18%) have been recorded in some soil samples. [84]
- 18. Applications Major applications of zinc include (numbers are given for the US)[88] 1. Galvanizing (55%) 2. Alloys (21%) 3. Brass and bronze (16%) 4. Miscellaneous (8%) Anti-corrosion and batteries Hot-dip handrail galvanized crystalline surface The metal is most commonly used as an anti-corrosion agent.[89] Galvanization, which is the coating of iron or steel to protect the metals againstcorrosion, is the most familiar form of using zinc in this way. In 2009 in the United States, 55% or 893 thousand tonnes of the zinc metal was used for galvanization. [88] Zinc is more reactive than iron or steel and thus will attract almost all local oxidation until it completely corrodes away.[90] A protective surface layer of oxide and carbonate (Zn5(OH)6(CO3)2) forms as the zinc corrodes.[91] This protection lasts even after the zinc layer is scratched but degrades through time as the zinc corrodes away.[91] The zinc is applied electrochemically or as molten zinc by hot-dip galvanizing or spraying. Galvanization is used on chain-link fencing, guard rails, suspension bridges, lightposts, metal roofs, heat exchangers, and car bodies.[10] The relative reactivity of zinc and its ability to attract oxidation to itself makes it an efficient sacrificial anode in cathodic protection (CP). For example, cathodic protection of a buried pipeline can be achieved by connecting anodes made from zinc to the pipe.[91] Zinc acts as the anode (negative terminus) by slowly corroding away as it passes electric current to the steel pipeline.[91][note 2] Zinc is
- 19. also used to cathodically protect metals that are exposed to sea water from corrosion.[92] A zinc disc attached to a ship's iron rudder will slowly corrode while the rudder stays unattacked.[90] Other similar uses include a plug of zinc attached to a propeller or the metal protective guard for the keel of the ship. With a standard electrode potential (SEP) of −0.76 volts, zinc is used as an anode material for batteries. (More reactive lithium (SEP −3.04 V) is used for anodes in lithium batteries ). Powdered zinc is used in this way in alkaline batteries and sheets of zinc metal form the cases for and act as anodes in zinc–carbon batteries.[93][94] Zinc is used as the anode or fuel of the zinc-air battery/fuel cell.[95][96][97] Alloys A widely used alloy which contains zinc is brass, in which copper is alloyed with anywhere from 3% to 45% zinc, depending upon the type of brass.[91] Brass is generally more ductile and stronger than copper and has superior corrosion resistance.[91] These properties make it useful in communication equipment, hardware, musical instruments, and water valves.[91] Cast brass microstructure at magnif ication 400x Other widely used alloys that contain zinc include nickel silver, typewriter metal, soft and aluminium solder, and commercial bronze.[4] Zinc is also used in contemporary pipe organs as a substitute for the traditional lead/tin alloy in pipes.[98] Alloys of 85–88% zinc, 4–10% copper, and 2–8% aluminium find limited use in certain types of machine bearings. Zinc is the primary metal used in making American one cent coins since 1982.[99] The zinc core is coated with a thin layer of copper to give the impression of a copper coin. In 1994, 33,200
- 20. tonnes (36,600 short tons) of zinc were used to produce 13.6 billion pennies in the United States.[100] Alloys of primarily zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries. [4] These alloys are marketed under the name Zamak.[101] An example of this is zinc aluminium. The low melting point together with the low viscosity of the alloy makes the production of small and intricate shapes possible. The low working temperature leads to rapid cooling of the cast products and therefore fast assembly is possible.[4][102] Another alloy, marketed under the brand name Prestal, contains 78% zinc and 22% aluminium and is reported to be nearly as strong as steel but as malleable as plastic.[4][103] This superplasticity of the alloy allows it to be molded using die casts made of ceramics and cement. [4] Similar alloys with the addition of a small amount of lead can be cold-rolled into sheets. An alloy of 96% zinc and 4% aluminium is used to make stamping dies for low production run applications for which ferrous metal dies would be too expensive.[104] In building facades, roofs or other applications in which zinc is used as sheet metal and for methods such as deep drawing, roll forming or bending, zinc alloys with titanium and copper are used.[105] Unalloyed zinc is too brittle for these kinds of manufacturing processes.[105] As a dense, inexpensive, easily worked material, zinc is used as a lead replacement. In the wake of lead concerns, zinc appears in weights for various applications ranging from fishing[106] to tire balances and flywheels.[107] Cadmium zinc telluride (CZT) is a semiconductive alloy that can be divided into an array of small sensing devices.[108] These devices are similar to an integrated circuit and can detect the energy of incoming gamma ray photons.[108] When placed behind an absorbing mask, the CZT sensor array can also be used to determine the direction of the rays. [108] Other industrial uses
- 21. Zinc oxide is used as a w hite pigment inpaints. Roughly one quarter of all zinc output in the United States (2009), is consumed in the form of zinc compounds;[88] a variety of which are used industrially. Zinc oxide is widely used as a white pigment in paints, and as a catalyst in the manufacture of rubber. It is also used as a heat disperser for the rubber and acts to protect its polymers from ultraviolet radiation (the same UV protection is conferred to plastics containing zinc oxide).[10] Thesemiconductor properties of zinc oxide make it useful in varistors and photocopying products.[109] The zinc zinc-oxide cycle is a two stepthermochemical process based on zinc and zinc oxide for hydrogen production.[110] Zinc chloride is often added to lumber as a fire retardant[111] and can be used as a wood preservative.[112] It is also used to make other chemicals.[111]Zinc methyl (Zn(CH3)2) is used in a number of organic syntheses.[113] Zinc sulfide (ZnS) is used in luminescent pigments such as on the hands of clocks, X-ray and television screens, and luminous paints.[114] Crystals of ZnS are used in lasers that operate in the mid-infrared part of the spectrum.[115] Zinc sulfate is a chemical in dyes and pigments.[111] Zinc pyrithione is used in antifouling paints.[116] Zinc powder is sometimes used as a propellant in model rockets.[117] When a compressed mixture of 70% zinc and 30% sulfur powder is ignited there is a violent chemical reaction.[117] This produces zinc sulfide, together with large amounts of hot gas, heat, and light.[117] Zinc sheet metal is used to make zinc bars.[118] 64Zn, the most abundant isotope of zinc, is very susceptible to neutron activation, being transmuted into the highly radioactive 65Zn, which has a half-
- 22. life of 244 days and produces intense gamma radiation. Because of this, Zinc Oxide used in nuclear reactors as an anti-corrosion agent is depleted of 64Zn before use, this is called depleted zinc oxide. For the same reason, zinc has been proposed as a salting material for nuclear weapons (cobalt is another, better-known salting material).[119] A jacket of isotopically enriched 64Zn would be irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, forming a large amount of 65Zn significantly increasing the radioactivity of the weapon's fallout.[119]Such a weapon is not known to have ever been built, tested, or used. [119] 65Zn is also used as a tracer to study how alloys that contain zinc wear out, or the path and the role of zinc in organisms.[120] Zinc dithiocarbamate complexes are used as agricultural fungicides; these include Zineb, Metiram, Propineb and Ziram.[121] Zinc naphthenate is used as wood preservative.[122] Zinc, in the form ofZDDP, is also used as an anti-wear additive for metal parts in engine oil.[123] Dietary supplement GNC zinc 50 mg tablets (AU) Zinc is included in most single tablet over-the-counter daily vitamin and mineral supplements.[124] Preparations include zinc oxide, zinc acetate, and zinc gluconate.[124] It is believed to possess antioxidant properties, which may protect against accelerated aging of the skin and muscles of the body;
- 23. studies differ as to its effectiveness.[125] Zinc also helps speed up the healing process after an injury.[125] It is also beneficial to the body's immune system. Indeed, zinc deficiency may have effects on virtually all parts of the human immune system.[126] The efficacy of zinc compounds when used to reduce the duration or severity of cold symptoms is controversial.[127] A 2011 systematic review concludes that supplementation yields a mild decrease in duration and severity of cold symptoms.[128] Zinc serves as a simple, inexpensive, and critical tool for treating diarrheal episodes among children in the developing world. Zinc becomes depleted in the body during diarrhea, but recent studies suggest that replenishing zinc with a 10- to 14-day course of treatment can reduce the duration and severity of diarrheal episodes and may also prevent future episodes for up to three months.[129] Zinc gluconate is one compound used for the delivery of zinc as a dietary supplement. The Age-Related Eye Disease Study determined that zinc can be part of an effective treatment for age-related macular degeneration.[130] Zinc supplementation is an effective treatment for acrodermatitis enteropathica, a genetic disorder affecting zinc absorption that was previously fatal to babies born with it.[50] Gastroenteritis is strongly attenuated by ingestion of zinc, and this effect could be due to direct antimicrobial action of the zinc ions in the gastrointestinal tract, or to the absorption of the zinc and re-release from immune cells (allgranulocytes secrete zinc), or both.[131][132][note 3] In 2011, researchers at John Jay College of Criminal Justicereported that dietary zinc supplements can mask the presence of drugs in urine. Similar claims have been made in web forums on that topic.[133] Although not yet tested as a therapy in humans, a growing body of evidence indicates that zinc may preferentially kill prostate cancer cells. Because zinc naturally homes to the prostate and because the prostate is accessible with relatively non-invasive procedures, its potential as a chemotherapeutic agent in
- 24. this type of cancer has shown promise.[134] However, other studies have demonstrated that chronic use of zinc supplements in excess of the recommended dosage may actually increase the chance of developing prostate cancer, also likely due to the natural buildup of this heavy metal in the prostate.[135] Topical use Further information: Zinc oxide#Medical Topical administration of zinc preparations include ones used on the skin, often in the form of zinc oxide. Zinc preparations can protect against sunburn in the summer and windburn in the winter.[50] Applied thinly to a baby's diaper area (perineum) with each diaper change, it can protect against diaper rash.[50] Zinc lactate is used in toothpaste to prevent halitosis.[136] Zinc pyrithione is widely applied in shampoos because of its anti-dandruff function.[137] Zinc ions are effective antimicrobial agents even at low concentrations.[138] Organic chemistry Addition of diphenylzinc to an aldehyde There are many important organozinc compounds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.[139][140][141][142] Among important applications is the Frankland-Duppa Reaction in which an oxalate ester(ROCOCOOR) reacts with an alkyl halide R'X, zinc and hydrochloric acid to the α-hydroxycarboxylic esters RR'COHCOOR,[143] the Reformatskii reaction which converts α-halo-esters and aldehydes to β-hydroxy-esters, the Simmons–Smith reaction in
- 25. which the carbenoid (iodomethyl)zinc iodide reacts with alkene(or alkyne) and converts them to cyclopropane, the Addition reaction of organozinc compounds to carbonyl compounds. The Barbier reaction(1899) which is the zinc equivalent of the magnesium Grignard reaction and is better of the two. In presence of just about any water the formation of the organomagnesium halide will fail whereas the Barbier reaction can even take place in water. On the downside organozincs are much less nucleophilic than Grignards, are expensive and difficult to handle. Commercially available diorganozinc compounds are dimethylzinc, diethylzinc and diphenylzinc. In one study[144][145] the active organozinc compound is obtained from much cheaper organobromine precursors: The Negishi coupling is also an important reaction for the formation of new carbon carbon bonds between unsaturated carbon atoms in alkenes, arenes and alkynes. The catalysts are nickel and palladium. A key step in the catalytic cycle is atransmetalation in which a zinc halide exchanges its organic substituent for another halogen with the palladium (nickel) metal center. The Fukuyama coupling is another coupling reaction but this one with a thioester as reactant forming a ketone. Biological role Zinc is an essential trace element, necessary for plants,[83] animals,[146] and microorganisms.[147] Zinc is found in nearly 100 specific enzymes[148] (other sources say 300), serves as structural ions in transcription factors and is stored and transferred in metallothioneins.[149] It is "typically the second most abundant transition metal in organisms" after iron and it is the only metal which appears in all enzyme classes.[83] In proteins, Zn ions are often coordinated to the amino acid side chains of aspartic acid, glutamic acid, cysteine and histidine. The theoretical and computational description of this zinc binding in proteins (as well as that of other transition metals) is difficult.[150] There are 2-4 grams of zinc[151] distributed throughout the human body. Most zinc is in the brain, muscle, bones, kidney, and liver, with the highest concentrations in the prostate and parts of the eye. [152] Semen is particularly rich in zinc, which is a key factor in prostate gland function and reproductive organ growth.[153]
- 26. In humans, zinc plays "ubiquitous biological roles".[1] It interacts with "a wide range of organic ligands",[1] and has roles in the metabolism of RNA and DNA, signal transduction, and gene expression. It also regulates apoptosis. A 2006 study estimated that about 10% of human proteins (2800) potentially bind zinc, in addition to hundreds which transport and traffic zinc; a similar in silico study in the plant Arabidopsis thaliana found 2367 zinc-related proteins.[83] In the brain, zinc is stored in specific synaptic vesicles by glutamatergic neurons[154] and can "modulate brain excitability".[1] It plays a key role in synaptic plasticity and so in learning.[155]However it has been called "the brain's dark horse"[154] since it also can be a neurotoxin, suggesting zinc homeostasis plays a critical role in normal functioning of the brain and central nervous system.[154] Enzymes Ribbon diagram of human carbonic anhydrase II, w ith zinc atom visible in the center Zinc f ingers help read DNA sequences.
- 27. Zinc is an efficient Lewis acid, making it a useful catalytic agent in hydroxylation and other enzymatic reactions.[148] The metal also has a flexible coordination geometry, which allows proteins using it to rapidly shift conformations to perform biological reactions.[156] Two examples of zinc-containing enzymes arecarbonic anhydrase and carboxypeptidase, which are vital to the processes of carbon dioxide (CO2) regulation and digestion of proteins, respectively.[157] In vertebrate blood, carbonic anhydrase converts CO2 into bicarbonate and the same enzyme transforms the bicarbonate back into CO2 for exhalation through the lungs.[158] Without this enzyme, this conversion would occur about one million times slower[159] at the normal blood pH of 7 or would require a pH of 10 or more.[160] The non-related β-carbonic anhydrase is required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and alcoholic fermentation.[161] Carboxypeptidase cleaves peptide linkages during digestion of proteins. A coordinate covalent bond is formed between the terminal peptide and a C=O group attached to zinc, which gives the carbon a positive charge. This helps to create a hydrophobicpocket on the enzyme near the zinc, which attracts the non-polar part of the protein being digested.[157] Other proteins Zinc serves a purely structural role in zinc fingers, twists and clusters.[162] Zinc fingers form parts of some transcription factors, which are proteins that recognize DNA base sequences during the replication and transcription of DNA. Each of the nine or ten Zn2+ ions in a zinc finger helps maintain the finger's structure by coordinately binding to four amino acids in the transcription factor.[159]The transcription factor wraps around the DNA helix and uses its fingers to accurately bind to the DNA sequence. In blood plasma, zinc is bound to and transported by albumin (60%, low-affinity) and transferrin (10%).[151] Since transferrin also transports iron, excessive iron reduces zinc absorption, and vice-versa. A similar reaction occurs with copper.[163] The concentration of zinc in blood plasma stays relatively constant regardless of zinc intake.[164] Cells in the salivary gland, prostate, immune system and intestine use zinc signaling as one way to communicate with other cells.[165]
- 28. Zinc may be held in metallothionein reserves within microorganisms or in the intestines or liver of animals.[166] Metallothionein in intestinal cells is capable of adjusting absorption of zinc by 15–40%.[167] However, inadequate or excessive zinc intake can be harmful; excess zinc particularly impairs copper absorpt ion because metallothionein absorbs both metals. [168] Reference ranges for blood tests, show ing zinc in purple at center-right Dietary intake Foods & spices containing zinc In the U.S., the Recommended Dietary Allowance (RDA) is 8 mg/day for women and 11 mg/day for men.[169] Median intake in the U.S. around 2000 was 9 mg/day for women and 14 mg/day in men.[170] Oysters, lobster[171] and red meats, especially beef, lamb and liver have some of the highest concentrations of zinc in food.[153]
- 29. The concentration of zinc in plants varies based on levels of the element in soil. When there is adequate zinc in the soil, the food plants that contain the most zinc are wheat (germ and bran) and various seeds (sesame, poppy, alfalfa, celery, mustard).[172] Zinc is also found in beans, nuts, almonds, whole grains,pumpkin seeds, sunflower seeds and blackcurrant.[173] Other sources include fortified food and dietary supplements, which come in various forms. A 1998 review concluded that zinc oxide, one of the most common supplements in the United States, and zinc carbonate are nearly insoluble and poorly absorbed in the body.[174] This review cited studies which found low plasma zinc concentrations after zinc oxide and zinc carbonate were consumed compared with those seen after consumption of zinc acetate and sulfate salts.[174] However, harmful excessive supplementation is a problem among the relatively affluent, and should probably not exceed 20 mg/day in healthy people,[175] although the U.S. National Research Council set a Tolerable Upper Intake of 40 mg/day.[176] For fortification, however, a 2003 review recommended zinc oxide in cereals as cheap, stable, and as easily absorbed as more expensive forms. [177] A 2005 study found that various compounds of zinc, including oxide and sulfate, did not show statistically significant differences in absorption when added as fortificants to maize tortillas.[178] A 1987 study found that zinc picolinate was better absorbed than zinc gluconate or zinc citrate. [179] However, a study published in 2008 determined that zinc glycinate is the best absorbed of the four dietary supplement types available.[180] Deficiency Main article: Zinc deficiency Zinc deficiency is usually due to insufficient dietary intake, but can be associated with malabsorption, acrodermatitis enteropathica, chronic liver disease, chronic renal disease, sickle cell disease, diabetes, malignancy, and other chronic illnesses.[2] Symptoms of mild zinc deficiency are diverse.[170] Clinical outcomes include depressed growth, diarrhea, impotence and delayed sexual maturation, alopecia, eye and skin lesions, impaired appetite, altered cognition, impaired host defense properties, defects in carbohydrate utilization, and reproductive teratogenesis. [164]Mild zinc deficiency
- 30. depresses immunity,[181] although excessive zinc does also.[151] Animals with a diet deficient in zinc require twice as much food in order to attain the same weight gain as animals given sufficient zinc. [114] Groups at risk for zinc deficiency include the elderly, children in developing countries, and those with renal insufficiency. The zinc chelator phytate, found in seeds and cereal bran, can contribute to zinc malabsorption.[2] Despite some concerns,[182] western vegetarians and vegans have not been found to suffer from overt zinc deficiencies any more than meat - eaters.[183] Major plant sources of zinc include cooked dried beans, sea vegetables, fortified cereals, soyfoods, nuts, peas, and seeds. [182] However, phytates in many whole-grains and fiber in many foods may interfere with zinc absorption and marginal zinc intake has poorly understood effects. There is some evidence to suggest that more than the US RDA (15 mg) of zinc daily may be needed in those whose diet is high in phytates, such as some vegetarians.[182] These considerations must be balanced against the fact that there is a paucity of adequate zinc biomarkers, and the most widely used indicator, plasma zinc, has poor sensitivity and specificity.[184] Diagnosing zinc deficiency is a persistent challenge.[1] Nearly two billion people in the developing world are deficient in zinc.[2] In children it causes an increase in infection and diarrhea, contributing to the death of about 800,000 children worldwide per year. [1] The World Health Organization advocates zinc supplementation for severe malnutrition and diarrhea.[185] Zinc supplements help prevent disease and reduce mortality, especially among children with low birth weight or stunted growth. [185] However, zinc supplements should not be administered alone, since many in the developing world have several deficiencies, and zinc interacts with other micronutrients.[186] Dreaming Some supplemental zinc users report an increase in vivid dreaming. Argriculture Zinc deficiency is crop plants' most common micronutrient deficiency; it is particularly common in high-pH soils.[187] Zinc-deficient soil is cultivated in the cropland of about half of Turkey and India, a third of China, and most of
- 31. Western Australia, and substantial responses to zinc fertilization have been reported in these areas.[83] Plants that grow in soils that are zinc-deficient are more susceptible to disease. Zinc is primarily added to the soil through the weathering of rocks, but humans have added zinc through fossil fuel combustion, mine waste, phosphate fertilizers, limestone, manure, sewage sludge, and particles from galvanized surfaces. Excess zinc is toxic to plants, although zinc toxicity is far less widespread.[83] Precautions Main article: Zinc toxicity Toxicity Although zinc is an essential requirement for good health, excess zinc can be harmful. Excessive absorption of zinc suppresses copper and iron absorption.[168] The free zinc ion in solution is highly toxic to plants, invertebrates, and even vertebrate fish.[188] The Free Ion Activity Model is well-established in the literature, and shows that just micromolar amounts of the free ion kills some organisms. A recent example showed 6 micromolar killing 93% of all Daphnia in water.[189] The free zinc ion is a powerful Lewis acid up to the point of being corrosive. Stomach acid contains hydrochloric acid, in which metallic zinc dissolves readily to give corrosive zinc chloride. Swallowing a post-1982 American one cent piece (97.5% zinc) can cause damage to the stomach lining due to the high solubility of the zinc ion in the acidic stomach.[190] There is evidence of induced copper deficiency at low intakes of 100–300 mg Zn/day; a recent trial had higher hospitalizations for urinary complications compared to placebo among elderly men taking 80 mg/day.[191] The USDA RDA is 11 and 8 mg Zn/day for men and women, respectively.[169] Even lower levels, closer to the RDA, may interfere with the utilization of copper and iron or adversely affect cholesterol. [168] Levels of zinc in excess of 500 ppm in soil interfere with the ability of plants to absorb other essential metals, such as iron and manganese. [84] There is also a condition called the zinc shakes or "zinc chills" that can be induced by the inhalation of freshly formed zinc oxide formed during the welding of galvanized materials.[114] Zinc is a common ingredient of denture cream
- 32. which may contain between 17 and 38 mg of zinc per gram. There have been cases of disability or even death due to excessive use of these products. [192] The U.S. Food and Drug Administration (FDA) has stated that zinc damages nerve receptors in the nose, which can cause anosmia. Reports of anosmia were also observed in the 1930s when zinc preparations were used in a failed attempt to prevent polio infections.[193] On June 16, 2009, the FDA said that consumers should stop using zinc-based intranasal cold products and ordered their removal from store shelves. The FDA said the loss of smell can be life-threatening because people with impaired smell cannot detect leaking gas or smoke and cannot tell if food has spoiled before they eat it.[194] Recent research suggests that the topical antimicrobial zinc pyrithione is a potent heat shock response inducer that may impair genomic integrity with induction of PARP-dependent energy crisis in cultured human keratinocytes and melanocytes.[195] Poisoning In 1982, the United States Mint began minting pennies coated in copper but made primarily of zinc. With the new zinc pennies, there is the potential for zinc toxicosis, which can be fatal. One reported case of chronic ingestion of 425 pennies (over 1 kg of zinc) resulted in death due to gastrointestinal bacterial and fungal sepsis, while another patient, who ingested 12 grams of zinc, only showed lethargy and ataxia (gross lack of coordination of muscle movements).[196] Several other cases have been reported of humans suffering zinc intoxication by the ingestion of zinc coins.[197][198] Pennies and other small coins are sometimes ingested by dogs, resulting in the need for medical treatment to remove the foreign body. The zinc content of some coins can cause zinc toxicity, which is commonly fatal in dogs, where it causes a severe hemolytic anemia, and also liver or kidney damage; vomiting and diarrhea are possible symptoms.[199] Zinc is highly toxic in parrots and poisoning can often be fatal.[200] The consumption of fruit juices stored in galvanized cans has resulted in mass parrot poisonings with zinc. [50]
- 33. inc is a metal. It is called an “es s ential trace element” becaus e very small amounts of zinc are neces s ary for human health. Zinc is used for treatment and prevention of zinc deficiency and its consequences, including stunted growth and acute diarrhea in children, and slow wound healing. It is also used for boosting the immune system, treating the common cold and recurrent ear infections, and preventing lower respiratory infections. It is also used for malaria and other diseases caused by parasites. Some people use zinc for an eye disease called macular degeneration, for night blindness, and for cataracts. It is also used for asthma; diabetes; high blood pressure; acquired immunodeficiency syndrome (AIDS); and skin conditions such aspsoriasis, eczema, and acne. Other uses include treating attention deficit-hyperactivity disorder (ADHD), blunted sense of taste (hypogeusia), ringing in the ears (tinnitus), severe head injuries, Crohn’s dis ease, Alzheimer’s disease, Down syndrome, Hansen’s disease, ulcerative colitis, peptic ulcers and promoting weight gain in people with eating disorders such as anorexia nervosa. Some people use zinc for benign prostatic hyperplasia (BPH), male infertility, erectile dysfunction (ED), weak bones (osteoporosis), rheumatoid arthritis, and muscle cramps associated with liver disease. It is also used for sickle cell disease and inherited disorders such as acrodermatitis enteropathica, thalassemia, and Wils on’s disease. Some athletes use zinc for improving athletic performance and strength. Zinc is also applied to the skin for treating acne, aging skin, herpes simplexinfections, and to speed wound healing. There is a zinc preparation that can be sprayed in the nostrils for treating the common cold. Zinc sulfate is used in products for eye irritation. Zinc citrate is used in toothpaste and mouthwash to prevent dental plaque formation and gingivitis. Note that many zinc products also contain another metal called cadmium. This is because zinc and cadmium are chemically similar and often occur together in nature. Exposure to high levels of cadmium over a long time can l ead to kidney failure. The concentration of cadmium in zinc-containing supplements can vary as much as 37-fold. Look for zinc-gluconate products. Zinc gluconate consistently contains the lowest cadmium levels. How does it work? Zinc is needed for the proper growth and maintenance of the human body. It is found in several systems and biological reactions, and it is needed for immune function, wound healing, blood clotting, thyroid function, and much more. Meats, seafood, dairy products, nuts, legumes, and whole grains offer relatively high levels of zinc. Zinc deficiency is not uncommon worldwide, but is rare in the US. Symptoms include slowed growth, low insulin levels, loss of appetite, irritability, generalized hair loss, rough and dry skin, slow wound healing, poor sense of taste and smell, diarrhea, and nausea. Moderate zinc deficiency is associated with disorders of the intestine which interfere with food absorption (malabsorption syndromes), alcoholism, chronic kidney failure, and chronic debilitating diseases. Zinc plays a key role in maintaining vision, and it is present in high concentrations in the eye. Zinc deficiency can alter vision, and severe deficiency can cause changes in the retina (the back of the eye where an image is focused). Zinc might also have effects against viruses. It appears to lessen symptoms of the rhinovirus (common cold), but res earchers can’t yet explain exactly how this works . In addition, there is s ome evidence that zinc has s ome antiviral activity against the herpes virus. Low zinc levels can be associated with male infertility, sickle cell disease, HIV, major depression, and type 2 diabetes, and can be fought by taking a zinc supplement.
- 34. Preventing and treating blood levels of zinc that are too low (zinc deficiency). Zinc deficiency may occur in severe diarrhea, conditions that make it hard for the bowel to absorb food, liver cirrhosis and alcoholism, after major surgery, and during long-term use of tube feeding in the hospital. Taking zinc by mouth or intravenously (by IV) helps to restore zinc levels to the right level. But as a rule, routine use of zinc supplements is not recommended. Likely Effective for: Reducing diarrhea in malnourished children, or in children who have low zinc levels. Severe zinc deficiency in children is common in developing countries. Treating Wils on’s dis ease, a rare genetic dis order. Possibly Effective for: Decreasing the length of time the common cold lasts, when taken by mouth as a lozenge. However, using zinc as a pill or a nos e s pray does n’t s eem to help prevent colds . Acne. Taking zinc by mouth or applying it to the skin in an ointment that also contains erythromycin seems to help clear up acne. Weak bones (osteoporosis). Low zinc intake seems to be linked to lower bone mass. Taking a zinc supplement in combination with copper, manganese, and calcium might also decrease bone loss in women who have passed menopause. Treating an eye disease called age-related macular degeneration (AMD) when taken with other medicines. Taking zinc by mouth in combination with antioxidant vitamins (vitamin C, vitamin E, and beta -carotene) might slow the worsening of advanced age-related macular degeneration (AMD). There is n’t enough inform ation to know if zinc plus antioxidants helps people with less advanced macular disease or prevents AMD. Taking zinc supplements alone does not seem to benefit people with exis ting AMD. Treating attention deficit-hyperactivity disorder (ADHD). Taking zinc by mouth in combination with conventional treatment might slightly improve symptoms of hyperactivity, impulsiveness, and socialization problems in some children with ADHD. But zinc might not improve attention span. Some research suggests that children with ADHD have lower zinc levels in their blood than children without ADHD. Other research suggests people with ADHD with lower zinc levels might not respond well enough to prescription medications for ADHD (stimulants). Studies using zinc for ADHD have taken place in the Middle East where zinc deficiency is relatively common compared to Wes tern countries . It’s not known if zinc would have the same potential benefits when used for ADHD in people from Western countries. Treating an inherited disorder called acrodermatitis enteropathica. Leprosy, when used with other medications. Herpes simplex virus when zinc preparations made for the skin are applied directly to the mouth or genitals. Promoting weight gain and improving depression in people with eating disorders such as anorexia nervosa. Treating hypogeusia, a rare condition where the sense of taste is abnormal. Preventing and treating stomach ulcers. Preventing complications related to sickle cell anemia in people who have low zinc levels. Preventing muscle cramps in people who have low zinc levels. Treating leg wounds in people with low zinc levels. As a mouthwash or toothpaste for preventing tartar and gingivitis. Improving healing of burns. Increasing vitamin A levels in underfed children or in children with low zinc levels. Preventing and treating pneumonia in undernourished children in developing countries.
- 35. Possibly Ineffective for: Preventing prostate cancer. Some preliminary research suggests that some men might benefit from taking zinc along with other vitamins and minerals for preventing prostate cancer. But other research suggests that taking zinc can increase the risk of developing prostate cancer and increase the risk of dying from prostate cancer. Raising blood iron levels in pregnant women, when taken with iron and folic acid supplements. Skin conditions including eczema, psoriasis, or hair loss. Many kinds of arthritis. Preventing or treating cataracts. Malaria in underfed children. Inflammatory bowel disease. “Ringing in the ears ” (tinnitus ). AIDS diarrhea-wasting syndrome. Preventing the flu. Increasing birth weight and gestation time in infants born to HIV-infected women. Insufficient Evidence for: Alzheimer’s dis ease. Some limited res earch has s hown zinc s upplements may s lightly s low the wors ening of symptoms in people with Alzheimer’s dis ease. Wrinkled skin. A skin cream containing 10% vitamin C as L-ascorbic acid and acetyl tyrosine, zinc sulfate, sodium hyaluronate, and bioflavonoids (Cellex-C High Potency Serum) applied for 3 months to facial skin aged by sun exposure seems to improve fine and coarse wrinkling, yellowing, roughness, and skin tone. Infections related to AIDS. There is some limited evidence that taking zinc supplements by mouth in combination with zidovudine (AZT, Retrovir, a component of Combivir) might prevent certain bacterial and yeast infections that can occur in people with AIDS because their immune system is less active than it should be. However, taking zinc supplements might lower the overall survival of people with AIDS. Male sexual problems. Taking zinc orally to treat male sexual problems caused by disease or medical treatment has produced varying results. Crohn’s dis ease. Ulcerative colitis. Diabetes. Treating the common cold when used as a nose spray. Asthma. Down syndrome. Recurrent ear infections. Preventing cancer. Head injury. Helping babies that are too small when born. Preventing esophageal cancer. Other conditions. More evidence is needed to rate zinc for these uses.
- 36. Zinc is LIKELY SAFE for most adults when applied to the skin, or when taken by mouth in amounts not larger than 40 mg per day. Routine zinc supplementation is not recommended without the advice of a healthcare professional. In some people, zinc might cause nausea, vomiting, diarrhea, metallic taste, kidney and stomach damage, and other side effects. Using zinc on broken skin may cause burning, stinging, itching, and tingling. Taking high amounts of zinc is LIKELY UNSAFE. High doses above the recommended amounts might cause fever, coughing, stomach pain, fatigue, and many other problems. Taking more than 100 mg of supplemental zinc daily or taking supplemental zinc for 10 or more years doubles the risk of developing prostate cancer. There is also concern that taking large amounts of a multivitamin plus a separate zinc supplement increases the chance of dying from prostate cancer. Taking 450 mg or more of zinc daily can cause problems with blood iron. Single doses of 10-30 grams of zinc can be fatal. Zinc nose sprays (Zicam, Cold-Eeze) are POSSIBLY UNSAFE. These products may cause loss of ability to smell. In June 2009, the US Food and Drug Administration (FDA) advised consumers not to use certain zinc-containing nose sprays (Zicam) after receiving over 100 reports of loss of smell. The maker of these zinc-containing nose sprays has also received several hundred reports of loss of smell from people who had used the products. Avoid using zinc nose sprays. Special Precautions & Warnings: Pregnancy and breast-feeding: Zinc is LIKELY SAFE for most pregnant and breast-feeding women when used in the recommended daily amounts (RDA). Pregnant women over 18 should not take more than 40 mg of zinc per day; pregnant women age 14 to 18 should not take more than 34 mg per day. Breast-feeding women over 18 should not take more than 40 mg of zinc per day; breast-feeding women age 14 to 18 should not take more than 34 mg per day. HIV (human immunodeficiency virus)/AIDS: Do not take zinc if you have HIV/AIDS. Zinc might shorten your life. interactions Antibiotics (Quinolone antibiotics) interacts with ZINC Zinc might decrease how much antibiotic the body absorbs. Taking zinc along with some antibiotics might decrease the effectiveness of some antibiotics. To avoid this interaction take zinc supplements at least 1 hour after antibiotics. Some of these antibiotics that might interact with zinc include ciprofloxacin (Cipro), enoxacin (Penetrex), norfloxacin (Chibroxin, Noroxin), sparfloxacin (Zagam), trovafloxacin (Trovan), and grepafloxacin (Raxar). Antibiotics (Tetracycline antibiotics) interacts with ZINC
- 37. Zinc can attach to tetracyclines in the stomach. This decreases the amount of tetracyclines that can be absorbed. Taking zinc with tetracyclines might decrease the effectiveness of tetracyclines. To avoid this interaction take zinc 2 hours before or 4 hours after taking tetracyclines. Some tetracyclines include demeclocycline (Declomycin), minocycline (Minocin), and tetracycline (Achromycin). Cisplatin (Platinol-AQ) interacts with ZINC Cisplatin (Platinol-AQ) is used to treat cancer. Taking zinc along with EDTA and cisplatin (Platinol -AQ) might increase the effects and side effects of cisplatin (Platinol -AQ). Penicillamine interacts with ZINC Penicillamine is used for Wilson's disease and rheumatoid arthritis. Zinc might decrease how much penicillamine your body absorbs and decrease the effectiveness of penicillamine. Minor Interaction Be watchful with this combination Amiloride (Midamor) interacts with ZINC Amiloride (Midamor) is used as a "water pill" to help remove excess water from the body. Another effect of amiloride (Midamor) is that it can increase the amount of zinc in the body. Taking zinc supplements with amiloride (Midamor) might cause you to have too much zinc in your body. The following doses have been studied in scientific research: BY MOUTH: For treating the common cold: one zinc gluconate or acetate lozenge, providing 9-24 mg elemental zinc, dissolved in the mouth every two hours while awake when cold symptoms are present. For diarrhea in malnourished or zinc-deficient children: 10-40 mg elemental zinc daily. For preventing and treating pneumonia in undernourished children in developing countries: 10-70 mg/day. For hypogeusia (sense of taste is abnormal): 25-100 mg zinc. For the eating disorder anorexia nervosa: 100 mg of zinc gluconate daily. For treating stomach ulcers: zinc sulfate 200 mg three times daily. For muscle cramps in zinc deficient people with liver disease: zinc sulfate 220 mg twice daily. For osteoporosis: 15 mg zinc combined with 5 mg manganese, 1000 mg calcium, and 2.5 mg copper has been used. For sickle cell disease: zinc sulfate 220 mg three times daily. To increase growth and weight gain in children with sickle cell disease who have not reached puberty: 10 mg elemental zinc per day. For treating attention deficit-hyperactivity disorder (ADHD) in children: doses of zinc sulfate 55 mg (15 mg elemental zinc) to 150 mg (40 mg elemental zinc) daily. For treating acne: 30-135 mg elemental zinc daily.
- 38. For treating age-related macular degeneration (AMD): elemental zinc 80 mg plus vitamin C 500 mg, vitamin E 400 IU, and beta-carotene 15 mg daily. The Institute of Medicine has established Adequate Intake (AI) levels of zinc for infants birth to 6 months is 2 mg/day. For older infants, children, and adults, Recommended Dietary Allowance (RDA) quantities of zinc have been established: infants and children 7 months to 3 years, 3 mg/day; 4 to 8 years, 5 mg/day; 9 to 13 years, 8 mg/day; girls 14 to 18 years, 9 mg/day; boys and men age 14 and older, 11 mg/day; women 19 and older, 8 mg/day; pregnant women 14 to 18, 13 mg/day; pregnant women 19 and older, 11 mg/day; lactating women 14 to 18, 14 mg/day; lactating women 19 and older, 12 mg/day. The typical North American male consumes about 13 mg/day of dietary zinc; women consume approximately 9 mg/day. The Tolerable Upper Intake Levels (UL) of zinc for people who are not receiving zinc under medical supervision: Infants birth to 6 months, 4 mg/day; 7 to 12 months, 5 mg/day; children 1 to 3 years, 7 mg/day; 4 to 8 years, 12 mg/day; 9 to 13 years, 23 mg/day; 14 to 18 years (including pregnancy and lactation), 34 mg/day; adults 19 years and older (including pregnancy and lactation), 40 mg/day. Different salt forms provide different amounts of elemental zinc. Zinc sulfate contains 23% elemental zinc; 220 mg zinc sulfate contains 50 mg zinc. Zinc gluconate contains 14.3% elemental zinc; 10 mg zinc gluconate contains 1.43 mg zinc. APPLIED TO THE SKIN: For acne vulgaris: zinc acetate 1.2% with erythromycin 4% as a lotion applied twice daily. For herpes simplex infections: zinc sulfate 0.25% applied 8 to 10 times daily or zinc oxide 0.3% with glycine applied every 2 hours while awake. zinc What can high-zinc foods do for you? Help balance blood sugar Stabilize your metabolic rate Prevent a weakened immune system Support an optimal sense of smell and taste What events can indicate a need for more high-zinc foods?
- 39. Impaired sense of taste or smell Lack of appetite Depression Growth failure in children Frequent colds and infections Calf's liver is an excellent source of zinc while very good sources of zinc include crimini mushrooms, shiitake mushrooms, venison, and spinach. World's Healthiest Foods rich in zinc FoodCals%Daily Value Venison21765.3% Lamb22930.6% Beef, grass-fed17527.2% Scallops12722.6% Sesame Seeds20618.6% Pumpkin Seeds18016.8% Oats16615.6%
- 40. Yogurt15414.5% Turkey15313.1% Shrimp11211.8% For serving size for specific foods, see Nutrient Rating Chart below at the bottom of this page. Description Function Deficiency Symptoms Toxicity Symptoms Cooking, storage and processing Factors that affect function Nutrient interaction Health conditions Food Sources Public Health Recommendations References Description What is zinc? Zinc is a micromineral needed in the diet on a daily basis, but only in very small amounts (50 milligrams or less). The other microminerals that all humans must get from food are arsenic, boron, cobalt, copper, chromium, fluorine, iodine, iron,manganese, molybdenum, nickel, silicon, vanadium, and zinc. The first research studies to demonstrate the zinc's important in the diet focused on the issue of growth. When foods did not supply sufficient amounts of zinc, young men in Iran and Egypt were found to have impaired overall growth as well as impaired sexual maturation. These initial studies on zinc reflected some of the key functions served by this mineral, including regulation of genetic activity and balance of carbohydrate metabolism and blood sugar.
- 41. How it Functions What is the function of zinc? Regulating genetic activities Zinc is an important regulator of many genetic activities. The cells of our body each have a special compartment called the nucleus, and inside the nucleus are approximately 100,000 genes. These genes provide instructions for the cell, and the cell has to decide which instructions to read. Zinc is essential for reading genetic instructions, and when diets do not contain foods rich in zinc, instructions get misread, or not read at all. (In biochemistry terms, the gene-reading process that requires zinc is called gene transcription.) Supporting blood sugar balance and metabolic rate Insulin, a hormone made by the pancreas, is often required to move sugar from our bloodstream into our cells. The response of our cells to insulin is called insulin response. When the foods in our diet do not provide us with enough zinc, insulin response decreases, and our blood sugar becomes more difficult to stabilize. Metabolic rate - the rate at which we create and use up energy - also depends on zinc for its regulation. When zinc is deficient in the diet, metabolic rate drops (along with hormonal output by our thyroid gland). Supporting smell and taste sensitivity Gustin is a small protein that is directly involved in our sense of taste. Zinc mus be linked to gustin in order for our sense of taste to function properly. Because of this relationship between zinc and taste, and because taste and smell are so closely linked in human physiology, impaired sense of taste and smell are common symptoms of zinc deficiency. Supporting immune function Many types of immune cells appear to depend upon zinc for optimal function. Particularly in children, researchers have studied the effects of zinc deficiency (and zinc supplementation) on immune response and number of white blood cells, including specific studies on T lymphocytes, macrophages, and B cells (all types of white blood cells). In these studies, zinc deficiency has been shown to compromise white blood cells numbers and immune response, while zinc supplementation has been shown to restore conditions to normal. Deficiency Symptoms What are deficiency symptoms for zinc?
- 42. Because of the link between zinc and the taste-related protein called gustin, impaired sense of taste and/or smell are common symptoms of zinc deficiency. Depression, lack of appetite, growth failure in children, and frequent colds and infections can also be symptomatic of insufficient dietary zinc. Toxicity Symptoms What are toxicity symptoms for zinc? Zinc toxicity has been reported in the research literature, and in 2000 the National Academy of Sciences set a tolerable upper limit (UI) of 40 milligrams for daily intake of zinc. (This limit applies to all individuals age 19 and over.) A metallic, bitter taste in the mouth can be indicative of zinc toxicity, as can stomach pain, nausea, vomiting, cramps, and diarrhea mixed with blood. Impact of Cooking, Storage and Processing How do cooking, storage, or processing affect zinc? Like most minerals, zinc is present in many different forms in food, and can vary greatly in its response to cooking and processing. In some foods, where a greater percent of zinc is found in water-soluble form and contact with water is great, high losses of zinc can occur. For example, when navy beans are cooked, 50% of the original zinc is lost. The processing of wheat is another example of the susceptibility of zinc to substantial loss. In 60% extraction wheat flour - the kind that is used to make over 90% of all breads, baked goods, and pastas sold in the U.S., almost 75% of the original zinc is lost. Factors that Affect Function What factors might contribute to a deficiency of zinc? In addition to dietary deficiency, problems in the digestive tract can contribute to zinc deficiency. These problems include irritable and inflammatory bowel disorders, as well as insufficient output by the pancreas that prevents proper digestion of food. Protein deficiency, and deficiency of one particular part of protein—the amino acid cysteine—can also contribute to zinc deficiency by preventing synthesis of transport and storage molecules that are used to shuttle and store zinc in the body.
- 43. Loss of zinc through chronic diarrhea or profuse sweating (as might occur with heavy physical labor or athletic training) can also contribute to deficiency of this mineral. Nutrient Interactions How do other nutrients interact with zinc? A Tolerable Upper Limit (UL) for zinc of 40 milligrams per day was set by the National Academy of Sciences in 2000 for all adults 19 years and older. The establishment of this limit was largely related to the ability of zinc—particularly supplemental zinc—to impair the status of other nutrients. The most important of these nutrients are copper and calcium. Even at moderate doses of 18-20 milligrams that can easily be obtained from food, zinc can compromise the body's supply of copper unless foods rich in copper are also included in the diet. When few foods high in calcium are included in the diet, high levels of zinc intake (usually obtained from supplements) can also decrease absorption of calcium from the intestine into the body. Although zinc is associated with these potential detrimental effects on copper and calcium, it is also supportive of other nutrients. The best studied of these nutrients in vitamin A. Without zinc, vitamin A cannot be effectively transported around the body, and cannot efficiently be mobilized when it is needed. Health Conditions What health conditions require special emphasis on zinc? Zinc may play a role in the prevention and/or treatment of the following health conditions: Acne Alcoholism Alopecia Alzheimer's disease Anorexia nervosa Atopic dermatitis Benign prostatic hypertrophy Cervical dysplasia Common cold Crohn's disease Diabetes Epilepsy Graves' disease Herpes simplex
- 44. HIV/AIDS Infertility (male) Inflammatory bowel diseases Influenza Macular degeneration Osteoarthritis PMS Psoriasis Rheumatoid arthritis Seborrheic dermatitis Senile cataracts Food Sources What foods provide zinc? Calf's liver is an excellent source of zinc. Crimini mushrooms, shiitake mushrooms, spinach, and venison are very good sources of zinc. Good sources include asparagus, chard, scallops, lamb, beef, maple syrup, shrimp, green peas, yogurt, oats, pumpkin seeds, sesame seeds, turkey, miso, and spelt. Introduction to Nutrient Rating System Chart In order to better help you identify foods that feature a high concentration of nutrients for the calories they contain, we created a Food Rating System. This system allows us to highlight the foods that are especially rich in particular nutrients. The following chart shows the World's Healthiest Foods that are either an excellent, very good, or good source of zinc. Next to each food name, you'll find the serving size we used to calculate the food's nutrient composition, the calories contained in the serving, the amount of zinc contained in one serving size of the food, the percent Daily Value (DV%) that this amount represents, the nutrient density that we calculated for this food and nutrient, and the rating we established in our rating system. For most of our nutrient ratings, we adopted the government standards for food labeling that are found in the U.S. Food and Drug Administration's "Reference Values for Nutrition Labeling."Read more background information and details of our rating system. World's Healthiest Foods ranked as quality sources of zinc
- 45. Food Serving Size Cals Amount (mg) DV (%) Nutrient Density World's Healthiest Foods Rating Venison 4 oz-wt 216.6 9.80 65.3 5.4 very good Spinach 1 cup cooked 41.4 1.37 9.1 4.0 very good Mushrooms - Crimini 1 cup 19.1 0.96 6.4 6.0 very good Mushrooms, Shiitake 87 g 29.6 0.90 6.0 3.7 very good Lamb 4 oz-wt 229.1 4.60 30.7 2.4 good Beef, grass-fed 4 oz 175.0 4.09 27.3 2.8 good Scallops 4 oz-wt 127.0 3.40 22.7 3.2 good Sesame Seeds 0.25 cup 206.3 2.79 18.6 1.6 good Pumpkin Seeds 0.25 cup 180.3 2.52 16.8 1.7 good Oats 1 cup cooked 166.1 2.34 15.6 1.7 good Yogurt 1 cup 154.3 2.18 14.5 1.7 good Turkey 4 oz-wt 153.1 1.97 13.1 1.5 good Shrimp 4 oz-wt 112.3 1.77 11.8 1.9 good Green Peas 1 cup raw 115.7 1.64 10.9 1.7 good Asparagus 1 cup raw 26.8 0.72 4.8 3.2 good Swiss Chard 1 cup cooked 35.0 0.58 3.9 2.0 good Maple Syrup 2 tsp 34.8 0.55 3.7 1.9 good Miso 1 tbs 34.2 0.44 2.9 1.5 good World's Healthiest Foods Rating Rule excellent DV>=75% OR Density>=7.6 AND DV>=10%
- 46. very good DV>=50% OR Density>=3.4 AND DV>=5% good DV>=25% OR Density>=1.5 AND DV>=2.5% Public Health Recommendations What are current public health recommendations for zinc? The Recommended Dietary Allowances for zinc, set in 1999 by the Institute of Medicine at the National Academy of Sciences, are as follows: Males and females, 0-6 months: 2 milligrams Males and females, 6-12 months: 3 milligrams Males and females, 1-3 years: 3 milligrams Males and females, 4-8 years: 5 milligrams Males and females, 9-13 years: 8 milligrams Males 14 years and older: 11 milligrams Females 14-18 years: 9 milligrams Females 19 years and older: 8 milligrams Pregnant females 18 years or younger: 12 milligrams Pregnant females 19 years and older: 11 milligrams Lactating females 18 years or younger: 13 milligrams Lactating females 19 years and older: 12 milligrams The National Academy of Sciences set a tolerable upper limit (UI) of 40 milligrams for daily intake of zinc. (This limit applies to all individuals age 19 and over.) For more details on this, see the Toxicity Symptoms section above. References Cerhan JR, Saag KG, Merlino LA et al. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003 Feb 15; 157(4):345-54. 2003. Chandra RK. Micronutrients and immune functions. Ann NY Acad Sci 1990;587:9-16. 1990. Dunn MA, Blalock TL, Cousins RJ. Metallothionein. Proc Soc Exp Biol Med 1987;187:107-119. 1987. Festa MD, Anderson HL, Dowdy RP, et al. Effect of zinc intake on copper excretion and retention in men. Am J Clin Nutr 1985;41:285-292. 1985. Forbes RM, Erdman JW Jr. Bioavailability of trace mineral elements. Ann Rev Nutr 1983;2:213-231. 1983. Groff JL, Gropper SS, Hunt SM. Advanced Nutrition and Human Metabolism. West Publishing Company, New York, 1995. 1995.
- 47. Hambridge KM, Casey CE, Krebs NF. Zinc. In: Mertz W. (Ed), Trace elements in human and animal nutrition. 5th Edition, Volume 2. Academic Press, Orlando, Florida, 1986. 1986. Meiners CR, Derise NL, Lau HC, et al. (1976). The content of nine mineral elements in raw and cooked mature dry legumes. J Arg Food Chem 1976;24:1126-1130. 1976. National Research Council. Recommended Dietary Allowances, 10th ed. Washington, DC: National Academy Press; 1989. 1989. Pedersen B, Eggum BO. The influence of milling on the nutritive value of flour from cereal grains. Part 2. Wheat. Qual Plant Plant Fds Hum Nutr 1983;33:51-61. 1983. Prasad AS, Cavdar AO, Brewer GJ, et al. Zinc deficiency in human subjects. Alan R Liss, Inc, New York, 1983. 1983. Smith JC Jr, McDaniel EG, Fan FF, et al. Zinc: a trace element in vitamin A metabolism. Science 1973;181:954. 1973. Solomons NW, Cousins RJ. Zinc. In: Solomons NW and Rosenberg IH. (Eds). Absorption and malabsorption of mineral nutrients. Alan R Liss, New York, 1984. 1984. Spencer H. Minerals and mineral interactions in human beings. J Am Diet Assoc 1986;86:864-867. 1986. Wada L, King JC. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. J Nutr 1986;116:1045-1053. 1986. Wu FY-H, Wu C-W. Zinc in DNA replication and transcription. Ann Rev Nutr 1987;7:251-272. 1987. Send this page to a friend...
- 48. inc is an essential mineral required by the body for maintaining a sense of smell, keeping a healthy immune system, building proteins, triggering enzymes, and creating DNA. Zinc also helps the cells in your body communicate by functioning as a neurotransmitter. A deficiency in zinc can lead to stunted growth, diarrhea, impotence, hair loss, eye and skin lesions, impaired appetite, and depressed immunity. Conversely, consuming too much zinc can lead to nausea, vomiting, loss of appetite, abdominal cramps, diarrhea, and headaches in the short term, and can disrupt absorption of copper and iron in the long term. If you have a zinc deficiency, then animal foods are better sources of zinc than plant foods. The current percent daily value (%DV) for Zinc is 15mg. Below is a list of the top ten foods highest in Zinc. #1: Oysters Depending on type and variety oysters provide 16-182mg of zinc per 100g serving. This accounts for 110%-1200% of the DV for zinc. The food highest in zinc is The Steamed Wild Eastern Oyster which provides 182 mg of zinc per 100g serving, or 76mg (509% DV) in 6 oysters, and 154mg (1029% DV) in a 3 ounce serving. Click to see complete nutrition facts. #2: Toasted Wheat Germ Packed in jars and sold toasted, wheat germ is great to sprinkle on top of any food. Try it on salads, rice, or steamed vegetables. Toasted wheat germ provides 17mg (112% DV) of zinc per 100g serving, which is 19mg (126% DV) per cup, and 1.2mg (8% DV) in a single tablespoon. Crude (untoasted) wheat germ provides 12mg (82% DV) of zinc per 100g serving, 14mg (94% DV) per cup, and 1mg (6% DV) per tablespoon. Click to see complete nutrition facts. Buy Wheat Germ from Amazon.com
- 49. #3: Veal Liver The liver of any animal is packed with vitamins and minerals and most commonly served as pâté or liverwurst. Veal liver has the most zinc with 12mg per 100g serving accounting for 81% of the DV, that is 8.98mg of zinc (60% DV) in a cooked slice of liver (80g). Liver is best prepared steamed or fried with onions and herbs. Click to see complete nutrition facts. #4: Low Fat Roast Beef Low fat beef shoulder, shank, and chuck all contain about 10mg (70% DV) of zinc per 100g serving, 18mg (119% DV) per pound, and 9mg (59% DV) in a 3 oz serving. If you buy pre-processed roast beef be sure to consult the nutrition facts about the cut and nutrients. Not all nutrition labels report zinc, so don't worry if you don't see it. Click to see complete nutrition facts. #5: Roasted Pumpkin and Squash Seeds A popular food in the Middle East and East Asia pumpkin and squash seeds contain about 10mg (70% DV) of zinc per 100g serving, 6.6mg (59% DV) per cup, and 3mg (19% DV) per ounce (~85 seeds). If you can't find these in your local supermarket you will surely find them in Middle Eastern or East Asian specialty stores. Alternatively, you can also save any pumpkin and squash seeds you have and roast them in your oven. The seeds are typically eaten by cracking the outer shell and eating the seed inside. Click to see complete nutrition facts. Seeds and Nuts with the Fewest Calories.
- 50. #6: Dried Watermelon Seeds Much like the pumpkin and squash, watermelon seeds are popular in the Middle East and East Asia and they should be in specialty stores catering to those cultures. It is also possible to just eat the seeds raw with the watermelon. You can shell them, or just chew them up whole. Dried watermelon seeds provide 10mg (70% DV) of zinc per 100g serving, 11mg (74 %DV) per cup, and 3mg (19% DV) per ounce. Click to see complete nutrition facts. #7: Dark Chocolate and Cocoa Powder Chocolate is showing more and more health benefits and dark chocolate is coming into vogue. Unsweetened baking chocolate provides 9.6mg (64% DV) of zinc per 100g serving (most bars are 50-100 grams). Cocoa powder will provide 6.8mg (45% DV) per 100g, or 5.4mg (39% DV) per cup, 0.3mg (2% DV) per tablespoon. Most milk chocolates provide around 2.3mg (15% DV) per 100g serving or 1mg (7% DV) per bar. Click to see complete nutrition facts. Buy Dark Chocolate from Amazon.com #8: Lamb (Mutton) Lamb is a common meat in the Middle East, Mediterranean, and most of Europe, but is increasing in popularity in the Americas. Lamb provides between 4.2- 8.7mg of zinc per 100g serving (28%-58% DV) depending on cut. That is up to 7.4mg (49% DV) in a 3 ounce serving (85 grams). Click to see complete nutrition facts.
- 51. #9: Peanuts Peanuts are a great source of zinc, 100 grams of oil roasted peanuts will provide 6.6mg (44% DV) of zinc, or 8.8mg (59% DV) in 1 cup chopped, 1.9mg (12% DV) per oz (~39 peanuts). Dry roasted peanuts will provide half as much zinc at 3.3mg (22% DV) per 100 gram serving, or 4.8mg (32% DV) per cup, and 1mg (6% DV) per oz. Click to see complete nutrition facts. Buy Peanuts from Amazon.com #10: Crab Almost any kind of crab will be a great source of zinc. Alaksa King crab in particular provides 7.6mg (51% DV) of zinc per 100 gram serving, which is 10.2mg (68% DV) in an average crab leg, and 6.5mg (43% DV) in a 3 ounce serving. Click to see complete nutrition facts. Other Zinc Rich Foods Alaska King Crab 7.6mg (51% DV) per 100 gram serving 10.2mg (68% DV) per leg (134 grams) 6.5mg (43% DV) per 3oz serving (85 grams) Click to see complete nutrition facts for Alaska King Crab Pork (Shoulder) 5mg (33% DV) per 100 gram serving 7.4mg (49% DV) per steak(147 grams) 4.2mg (28% DV) per 3oz serving (85 grams) Click to see complete nutrition facts for Pork Shoulder Fortified Cereals (Varies By Brand) 52mg (345% DV) per 100 gram serving 15.5mg (103% DV) per cup Click to compare nutrition facts for various cereals
- 52. Chicken Leg (Roasted) 2.9mg (19% DV) per 100 gram serving 2.7mg (18% DV) per leg(95 grams) 4mg (27% DV) per cup (140 grams) Click to see complete nutrition facts for Chicken Legs Pork Tenderloin 3mg (20% DV) per 100 gram serving 2.2mg (14% DV) per chop (73 grams) 2.5mg (17% DV) per 3oz serving(85 grams) Click to see complete nutrition facts for Pork Tenderloin Lobster 2.9mg (19% DV) per 100 gram serving 4.2mg (28% DV) per cup (145 grams) 2.5mg (17% DV) per 3oz serving(85 grams) Click to see complete nutrition facts for Cooked Lobster Baked Beans 1.4mg (9% DV) per 100 gram serving 3.5mg (24% DV) per cup (253 grams) Click to see complete nutrition facts for Baked Beans Dry Roasted Cashews 5.6mg (37% DV) per 100 gram serving 7.7mg (51% DV) per cup (137 grams) 1.6mg (10% DV) per 1oz serving(28 grams) Click to see complete nutrition facts for Dry Roasted Cashews Low Fat Yogurt with Fruit 0.7mg (4% DV) per 100 gram serving 1.6mg (11% DV) per cup (245 grams) 0.8mg (5% DV) per 1/2 cup (113 grams) Click to see complete nutrition facts for Low Fat Yogurt with Fruit Chickpeas (Garbanzo Beans) 1.5mg (10% DV) per 100 gram serving 2.5mg (17% DV) per cup (164 grams) 1.3mg (9% DV) per 1/2 cup (82 grams) Click to see complete nutrition facts for Chickpeas (Garbanzo Beans) Almonds 3.5mg (24% DV) per 100 gram serving 4.9mg (33% DV) per cup (138 grams) 1mg (7% DV) per 1 ounce serving (28 grams) Click to see complete nutrition facts for Almonds Milk 0.4mg (3% DV) per 100 gram serving 1mg (7% DV) per cup (244 grams) 3.9mg (26% DV) per 1 quart serving (976 grams) Click to see complete nutrition facts for Milk Chicken Breast 1mg (7% DV) per 100 gram serving 1.4mg (9% DV) per cup (140 grams) 0.9mg (6% DV) for half a chicken breast (86 grams) Click to see complete nutrition facts for Chicken Breast
- 53. Cheddar Cheese 3.1mg (21% DV) per 100 gram serving 3.5mg (23% DV) per cup (113 grams) 0.9mg (6% DV) per ounce(oz) (28 grams) Click to see complete nutrition facts for Cheddar Cheese Mozzarella 2.9mg (19% DV) per 100 gram serving 3.3mg (22% DV) per cup (112 grams) 0.8mg (5% DV) per ounce(oz) (28 grams) Click to see complete nutrition facts for Mozzarella Kidney Beans 1mg (7% DV) per 100 gram serving 1.9mg (13% DV) per cup (177 grams) 0.1mg (1% DV) per tablespoon (11 grams) Click to see complete nutrition facts for Kidney Beans Green Peas 1.2mg (8% DV) per 100 gram serving 1.9mg (13% DV) per cup (160 grams) 1.5mg (6% DV) per half cup (80 grams) Click to see complete nutrition facts for Green Peas Sesame Seeds (Tahini) 10.5mg (70% DV) per 100 gram serving 1.5mg (10% DV) per tablespoon (14 grams) 2.9mg (20% DV) per 1 ounce serving (28 grams) Click to see complete nutrition facts for Sesame Seeds (Tahini) Flat Fish (Flounder or Sole) 0.6mg (4% DV) per 100 gram serving 0.8mg (5% DV) per fillet (127 grams) 0.5mg (4% DV) per 3 ounce serving (85 grams) Click to see complete nutrition facts for Flat Fish (Flounder or Sole) Health Benefits of Zinc Healthy Immune Function - Even mild to moderate zinc deficiency can depress the immune system through impaired macrophage and neutrophil functions, and associated effects.3 Zinc is also essential for creation and activation of T-lymphocytes. 4,5 Further, low levels of zinc have been associated with increased susceptibility to pneumonia and other infections in children and the elderly.6-9 Alleviation of the Common Cold (*Controversial) - There are conflicting studies as to weather or not zinc supplements can alleviate symptoms of the common cold and shorten its duration. At least one study confirms decreased duration of cold symptoms compared to a control,10 however, other studies report no effect.11,12 Since no harm is reported, increasing zinc intake could only help.
- 54. Healing of Cuts and Wounds - Zinc is essential for healthy skin and maintenance of mucosal membranes. Adequate levels of zinc is necessary for proper wound healing.13 Reduced Severity and Duration of Diarrhea - Studies show that increased intake of zinc can reduce duration and severity of diarrhea in undernourished children with infections.14-17 Prevention and Reduction of Age-Related Eye Damage - High dietary intake of zinc, as well as vitamins C, E, and beta-carotene, has been associated with reduced age-related macular deneration in the edlerly.18 High Risk Groups for a Zinc Deficiency Alcoholics - 30-50% of alcoholics have low levels of zinc because alcohol decreases zinc absorption and increases urinary secretion of zinc. Vegetarians - The bio-availability of zinc is higher in meats and thus more easily absorbed. Further legumes and whole grains contain phylates which bind zinc and inhibit absorption. (See lists of fruits and vegetables high in zinc.) Pregnant and Lactating Women - A developing fetus requires a high amount of zinc, likewise, there is a high amount of zinc lost through breast milk after birth. Older Infants who are Exclusively Breastfed - Infants older than 6 months should eat age-appropriate foods which provide zinc as the amount in breast milk is no longer ample. People with Sickle Cell Disease - For unknown reasons 44% of children, and 60-70% of adults with sickle cell disease have low levels of zinc. People with Gastrointestinal and Other Diseases - Gastrointestinal surgery, Crohn's disease, ulcerative colitis, short bowel syndrome, and other digestive diseases can all decrease zinc absorption and increase zinc loss from the body. People consuming high doses of Iron Supplements - Iron can interfere with zinc absorption, to reduce this effect, iron suppliments should be taken between meals to allow time for zinc to be absorbed properly. People taking Diuretics - Thiazide diuretics such as chlorthalidone (Hygroton®) and hydrochlorothiazide (Esidrix® and HydroDIURIL®) can increase zinc excretion by 60%, and over the long term, deplete body tissues of zinc stores. Be sure to consult your doctor or clinician to monitor your zinc level if you are taking
- 55. these diuretics for a sustained period of time, and be sure to eat more zinc rich foods. Recipes High in Zinc Buckwheat (How to Cook Buckwheat) Teff (How to Cook Teff) Blackberry Salad Warnings Oysters, liver, lamb, and cheese are high cholesterol foods which should be eaten in moderate amounts and avoided by people at risk of heart disease or stroke. Sesame Seeds, Pumpkin Seeds, Squash Seeds, and Peanuts are high calorie foods and should be eaten in moderate amounts by people with a high body mass index. Zinc suppliments have adverse reactions with the following medications: o Antibiotics - Certain antibiotics like quinolone antibiotics (such as Cipro®) and tetracycline antibiotics (such as Achromycin® and Sumycin®) inhibit the absorption of zinc in the digestive tract. o Penicillamine - Zinc reduces the absorption of Penicillamine, which is used by people suffering from rheumatoid arthritis. Taking zinc suppliments two hours before or after intake of Penicillamine solves this problem. Read more at http://www.healthaliciousness.com/articles/zinc.php#1K38W07A62UvJkri.99