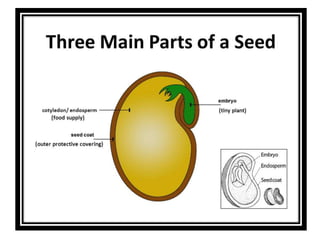
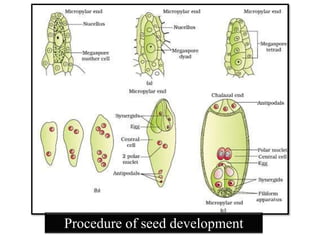
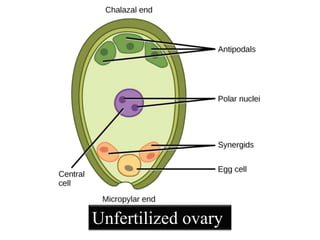
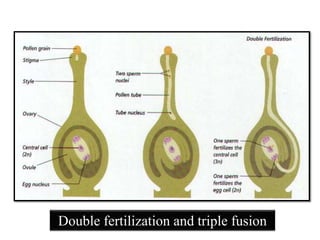
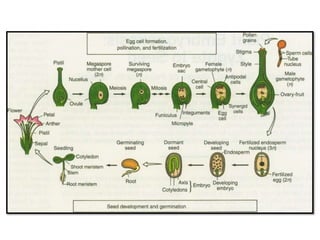
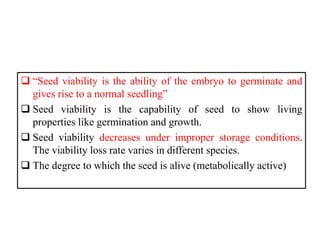
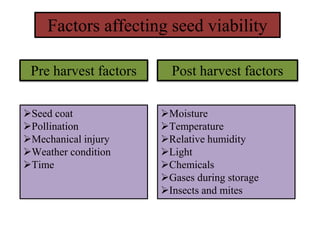
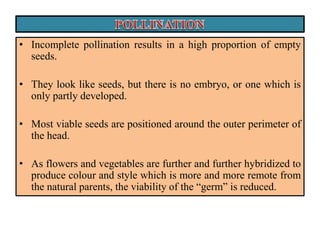
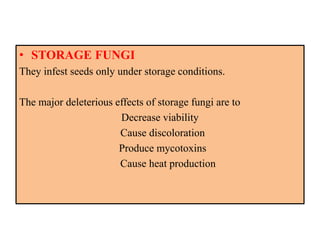
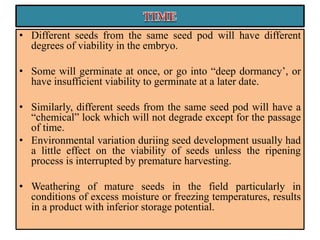
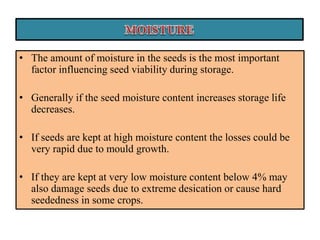
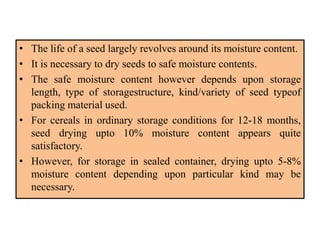
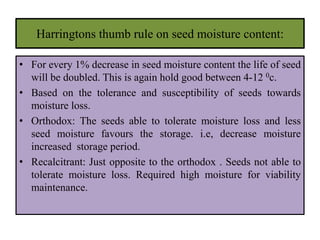
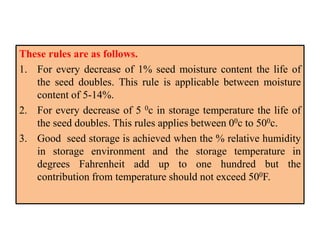
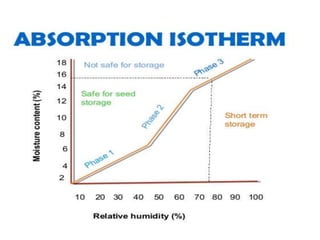
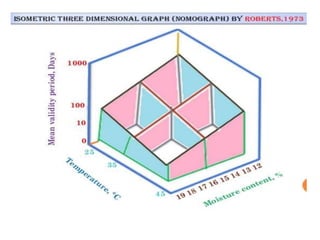
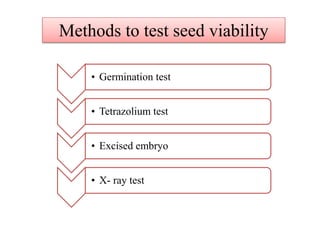
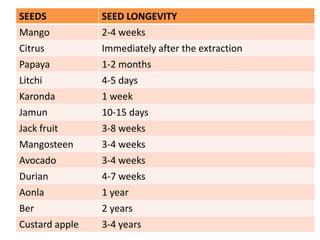
This document provides an overview of seed viability and factors that affect it. It defines what a seed is and describes seed development. Seed viability is the ability of a seed to germinate and produce a normal seedling. It is highest at physiological maturity and declines over time. Factors like moisture content, temperature, relative humidity, mechanical damage, and storage fungi can impact seed viability during development and post-harvest storage. The document also discusses methods to test seed viability, such as germination tests and tetrazolium tests.