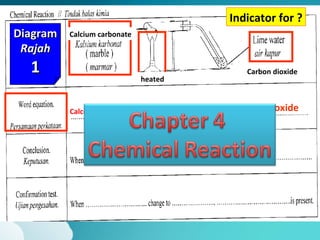
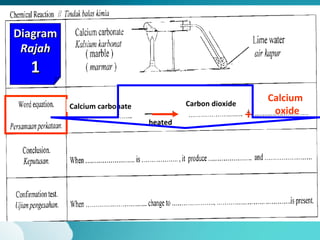
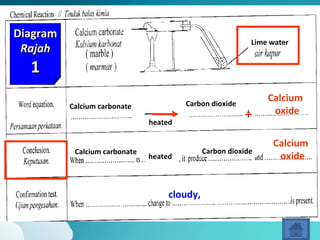
This document describes three diagrams that show the reaction between calcium carbonate and heat. In the first diagram, calcium carbonate is heated and produces calcium oxide and carbon dioxide. In the second diagram, the same reaction occurs and produces limewater that turns cloudy, indicating the presence of calcium ions. In the third diagram, calcium carbonate is directly heated to produce calcium oxide and carbon dioxide. The document also provides definitions and properties of acids and alkalis, including that acids and alkalis only react in the presence of water and have characteristic tastes or properties.