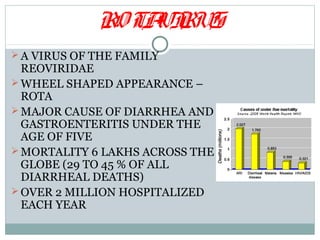
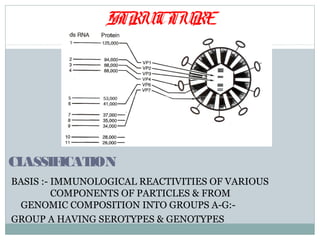
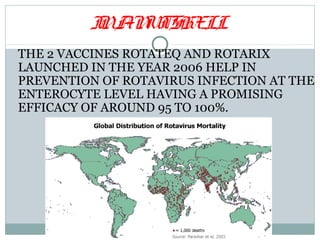
This document discusses rotavirus, a major cause of diarrhea and gastroenteritis in children under 5. It causes over 6 lakh deaths globally each year. The virus has a wheel-shaped appearance and belongs to the Reoviridae family. It replicates in the cytoplasm of intestinal cells. Two vaccines, Rotarix and RotaTeq, launched in 2006 help prevent rotavirus infection and have 95-100% efficacy. Challenges to vaccine introduction include uncertain demand, insufficient supply, high prices, and emergence of new viral strains.