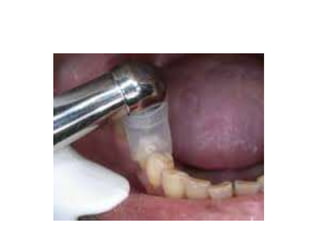
This document discusses root canal irrigation and intracanal medicaments. It outlines the objectives and characteristics of an ideal irrigation system, including removing debris, being bactericidal, and dissolving tissue. EDTA and sodium hypochlorite are described as common irrigation solutions used to remove the smear layer. Factors influencing irrigation efficacy and methods of irrigant delivery are covered. Potential complications of sodium hypochlorite accidents and their treatment are summarized. Finally, the document briefly discusses root canal medication and categories of intracanal medicaments.