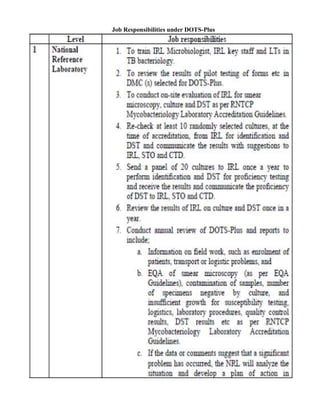
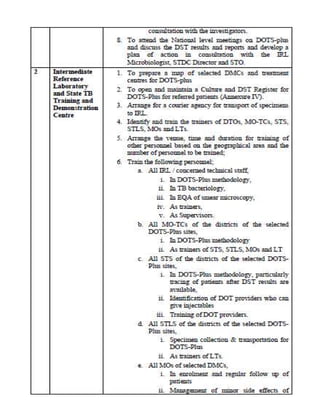
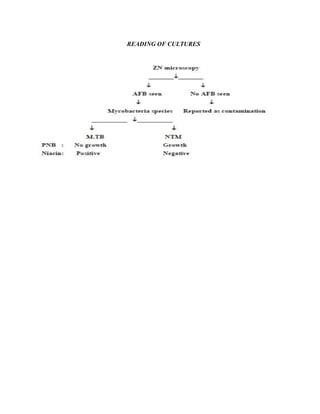
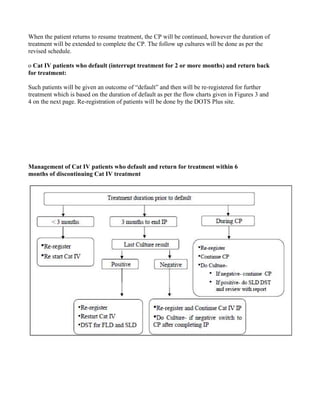
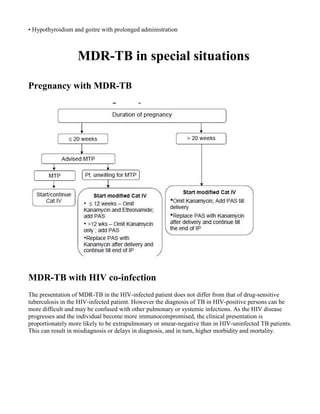
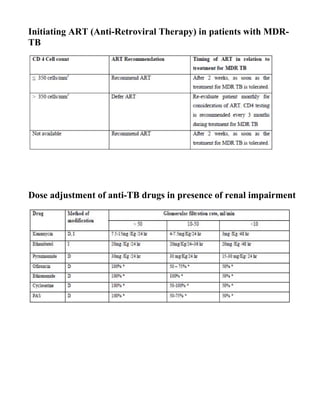
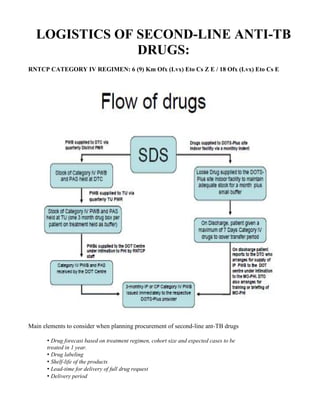
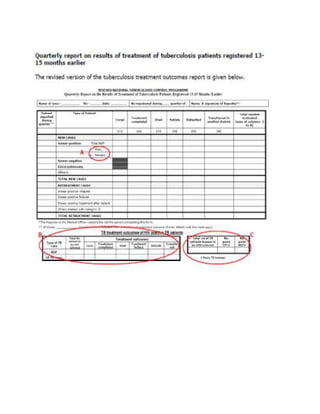
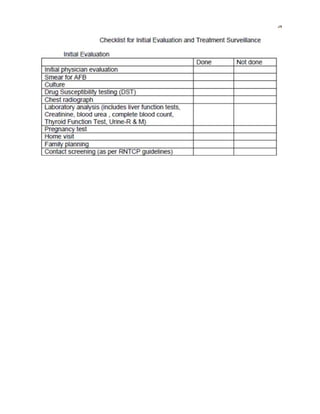
This document provides guidelines for the Revised National Tuberculosis Control Programme (RNTCP) in India to address multidrug-resistant TB (MDR-TB). It outlines a framework for integrating MDR-TB diagnosis, treatment, and management into existing DOTS programs. Key points include establishing quality-assured laboratories for culture and drug susceptibility testing; treating MDR-TB patients according to international guidelines using standardized second-line drug regimens and directly observed therapy; ensuring an uninterrupted supply of quality-assured drugs; and implementing a standardized recording and reporting system. The guidelines aim to make MDR-TB diagnosis and treatment available nationwide by expanding services gradually over time.










![LABORATORY ASPECTS:
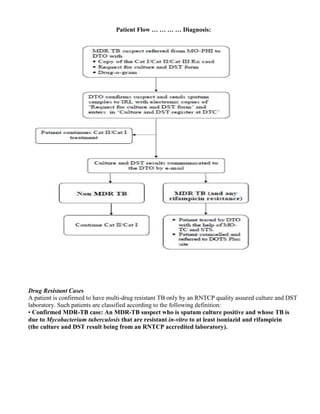
Drug-resistant case: A patient whose TB is due to tubercle bacilli that are resistant in vitro to at least
one anti-TB drug according to accepted laboratory methods in an RNTCP accredited laboratory.
Mono-resistance: A patient whose TB is due to tubercle bacilli that are resistant in vitro to exactly
on anti-TB drug in an RNTCP accredited laboratory.
Poly-resistance: A patient whose TB is due to tubercle bacilli that are resistant in-vitro to more
than one anti-TB drug, except not both isoniazid and rifampicin in an RNTCP accredited
laboratory.
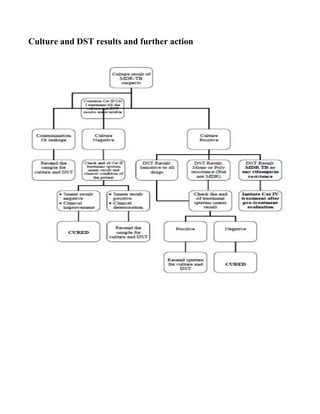
MDR-TB case: An MDR-TB suspect who is sputum culture positive and whose TB is due to
Mycobacterium tuberculosis that are resistant in-vitro to isoniazid and rifampicin with or without
other anti-tubercular drugs based on DST results from an RNTCP accredited Culture & DST
Laboratory.
Organization and development of the laboratory network
RNTCP has a three tier laboratory network based on the designated microscopy centres (DMCs) covering 1
lakh populations and providing sputum smear microscopy services, and IRLs (undertaking training,
external quality assessment [EQA] of sputum smear microscopy network in the districts and DMCs, and
culture and DST for first line drugs for M. tuberculosis), and NRLs (undertaking training, EQA of sputum
smear microscopy network in the states allotted to them, and culture and DST for first and second line
drugs for M. tuberculosis).](https://image.slidesharecdn.com/rntcpfinal-120115083844-phpapp02/85/RNTCP-by-Tikal-13-320.jpg)