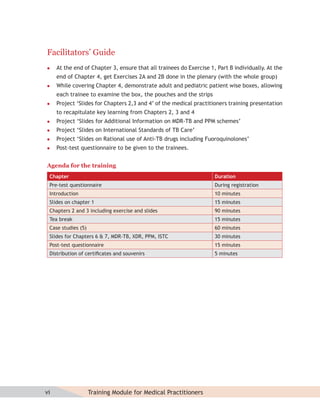
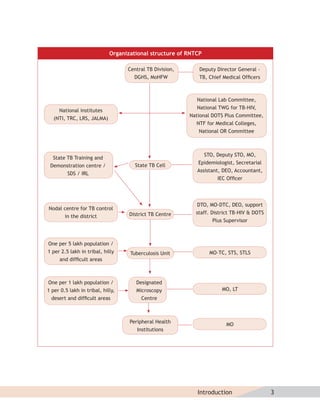
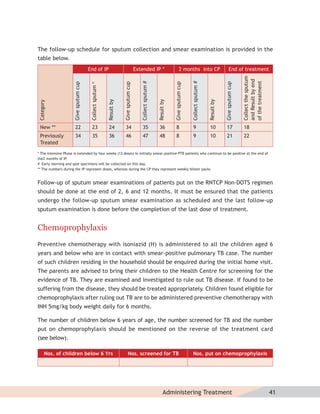
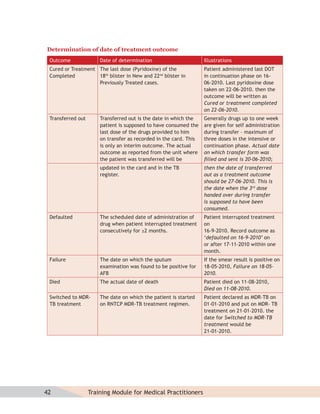
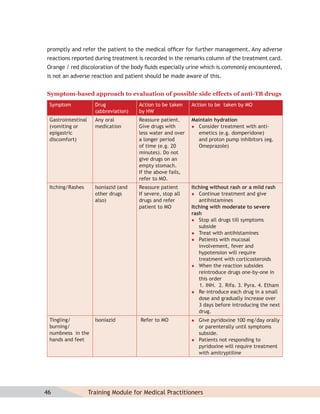
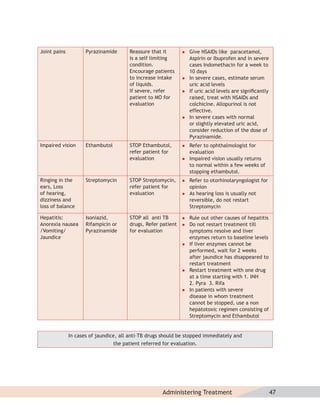
This document provides information about the Revised National Tuberculosis Control Programme (RNTCP) in India. It discusses:
- The goals and components of RNTCP, which implements the WHO-recommended Directly Observed Treatment, Short-course (DOTS) strategy.
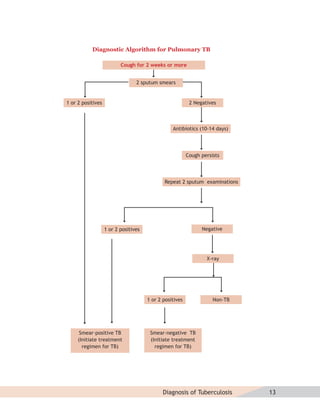
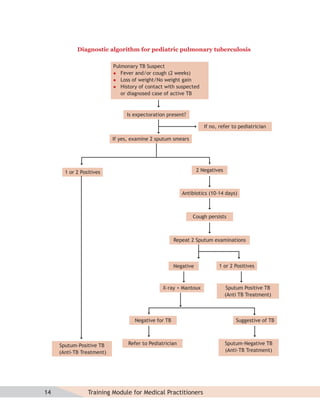
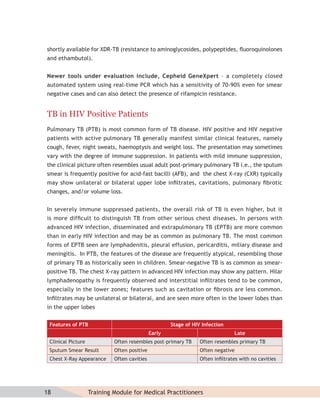
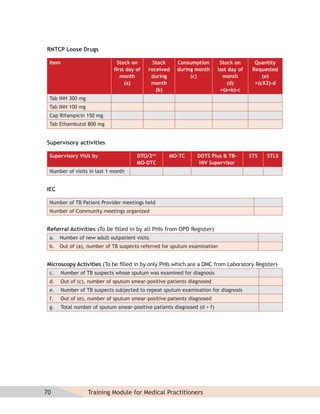
- Guidelines for diagnosing tuberculosis, including identifying suspects, conducting sputum examinations at Designated Microscopy Centers, and using tools like sputum microscopy, x-rays, and newer rapid diagnostic tests.
- Protocols for administering tuberculosis treatment under RNTCP, including categorizing patients, understanding treatment regimens and monitoring, and ensuring treatment adherence through mechanisms like DOTS.
- Efforts to expand universal access to tuberculosis



















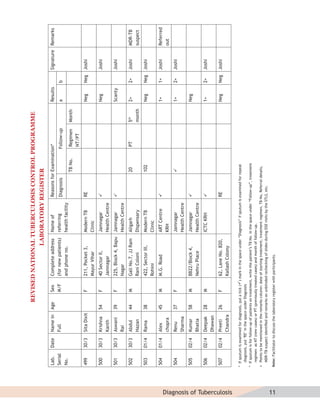



























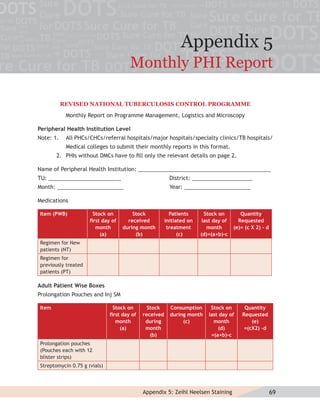
![MDR-TB suspects may include additional groups as the program expands in future. Check
the status with the local program manager.
Diagnosis of MDR-TB
For patients in whom drug resistance is suspected, diagnosis of MDR-TB should be done through
culture and drug susceptibility testing from a quality-assured laboratory. On being diagnosed
as an MDR-TB case, the patient will be referred to a designated state level DOTS-Plus site.
These sites are specialized centres which are limited in number. At least one such center is
expected to be in each state which has ready access to an RNTCP accredited culture and
DST laboratory. The DOTS-Plus site will be supported by qualified staff available to manage
patients using the second-line RNTCP MDR-TB regimen given under daily DOT and standardized
follow-up protocols. There will be a mechanism to deliver ambulatory DOT after an initial
short period of up to one week of in-patient care to stabilize the patient on second line drug
regimen. Logistics and standardized information will be made available in such places.
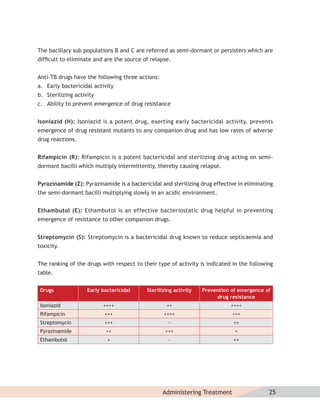
RNTCP MDR-TB Treatment Regimen
The RNTCP is using a standardised treatment regimen (STR), comprising of 6 drugs
(kanamycin [Km], levofloxacin(lvx), ethionamide [Eto], pyrazinamide [Z], ethambutol [E]
and cycloserine [Cs]) during 6-9 months of an Intensive Phase, and 4 drugs (lvx, Eto, E and
Cs) during the 18 months of the Continuation Phase. p-aminosalicylic acid (PAS) is included in
the regimen as a substitute drug if any of the drugs (bactericidal—kanamycin/ethionamide)
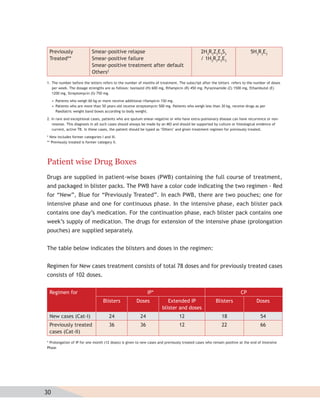
cannot be tolerated. Dosages of drugs are based upon three weight bands.
Drug 16-25 Kg 26-45 Kg > 45 Kg
Km 500 mg 500 mg 750 mg
LVX 200 mg 500 mg 750 mg
Eto 375 mg 500 mg 750 mg
E 400 mg 800 mg 1000 mg
Z 500 mg 1250 mg 1500 mg
Cs 250 mg 500 mg 750 mg
PAS 5g 10 g 12 g
Pyridoxine 50 mg 100 mg 100 mg
54 Training Module for Medical Practitioners](https://image.slidesharecdn.com/trainingmoduleformedicalpractitioners-120829120554-phpapp02/85/Training-module-for-medical-practitioners-66-320.jpg)






































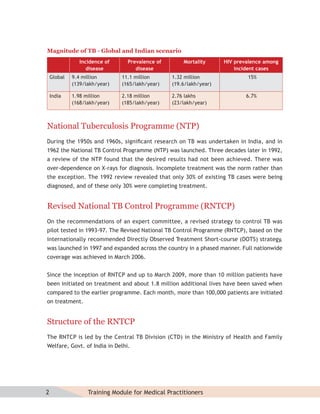
![Revised National Tuberculosis Control Programme
Memorandum of Understanding (MoU) for the participation of
Non-Governmental Organisations (NGOs)/Private Providers
1. Parties
This is to certify that ______________ _______________________
[Name of NGO/Private Provider] hence forth referred to as NGO/PP, has been enrolled as
an NGO/Private Provider in the District of ______________________ [Name of District] for
performance of the following activities in accordance with
RNTCP policy; under the schemes listed below:
(Please tick the appropriate scheme. If a NGO/PP opts for more than one scheme, tick
accordingly on a single MoU. Strike out whichever is not applicable).
i. TB advocacy, communication, and social mobilization scheme
ii. Sputum collection centre/s Scheme
iii. Sputum pickup and transportation Scheme
iv. Designated Microscopy Centre Scheme
v. Laboratory Technician Scheme
vi. Culture-DST Scheme
vii. Treatment Adherence Scheme
viii. Urban Slum Scheme
ix. Scheme for Tuberculosis Unit
x. TB-HIV Scheme
2. Period of Cooperation:
The NGO/Private Provider agrees to perform all activities outlined in the RNTCP NGO/
Private Provider schemes. The duration of cooperation will be from ___/___/_____ (dd/
mm/yyyy) to ___/____/____ (dd/mm/yyyy). In case of poor performance and non-diligence,
the contract can be terminated by the DHS at any time without prior notice.
106 Training Module for Medical Practitioners](https://image.slidesharecdn.com/trainingmoduleformedicalpractitioners-120829120554-phpapp02/85/Training-module-for-medical-practitioners-118-320.jpg)




![Duplicate
Revised National Tuberculosis Control Programme
Memorandum of Understanding (MoU) for the participation of
Non-Governmental Organisations (NGOs)/Private Providers
1. Parties
This is to certify that ______________ _______________________
[Name of NGO/Private Provider] hence forth referred to as NGO/PP, has been enrolled as
an NGO/Private Provider in the District of ______________________ [Name of District] for
performance of the following activities in accordance with
RNTCP policy; under the schemes listed below:
(Please tick the appropriate scheme. If a NGO/PP opts for more than one scheme, tick
accordingly on a single MoU. Strike out whichever is not applicable).
i. TB advocacy, communication, and social mobilization scheme
ii. Sputum collection centre/s Scheme
iii. Sputum pickup and transportation Scheme
iv. Designated Microscopy Centre Scheme
v. Laboratory Technician Scheme
vi. Culture-DST Scheme
vii. Treatment Adherence Scheme
viii. Urban Slum Scheme
ix. Scheme for Tuberculosis Unit
x. TB-HIV Scheme
2. Period of Cooperation:
The NGO/Private Provider agrees to perform all activities outlined in the RNTCP NGO/
Private Provider schemes. The duration of cooperation will be from ___/___/_____ (dd/
mm/yyyy) to ___/____/____ (dd/mm/yyyy). In case of poor performance and non-diligence,
the contract can be terminated by the DHS at any time without prior notice.](https://image.slidesharecdn.com/trainingmoduleformedicalpractitioners-120829120554-phpapp02/85/Training-module-for-medical-practitioners-123-320.jpg)