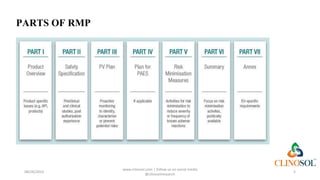
The document outlines the risk management process in pharmacovigilance, emphasizing its role in promoting the safe use of medicines and protecting patient health. It includes activities for identifying risks, assessing safety profiles, and implementing mitigation measures, all integral to the risk management plan required during drug authorization. The document also describes the legal framework and ongoing evaluation needed to minimize risks associated with drug products post-marketing.