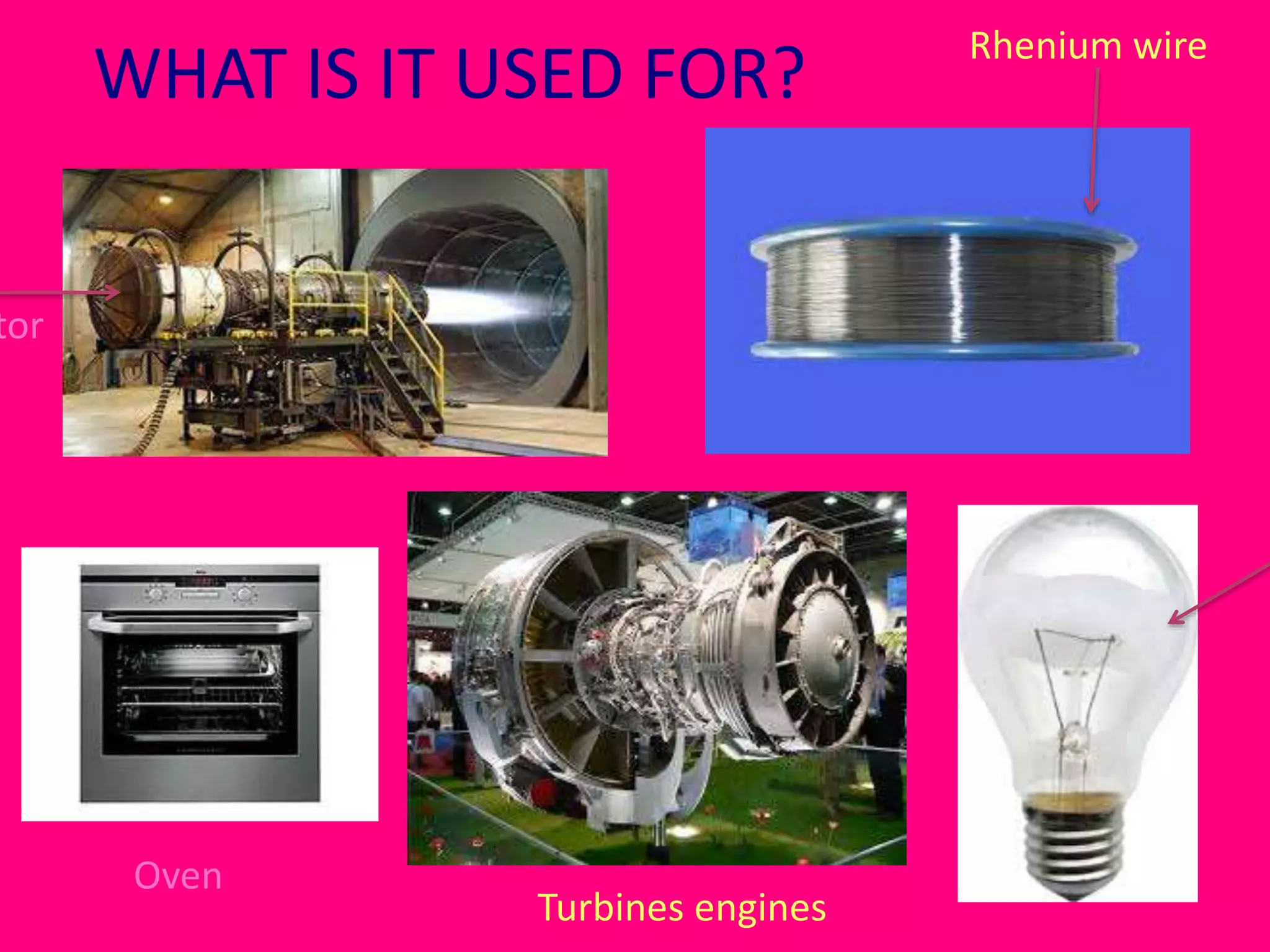
Rhenium is a rare, silvery-grey metal that was discovered in 1925 by Ida Noddack, Walter Noddack, and Otto Berg in Germany. It has a very high melting point of over 3000°C and does not readily react with oxygen or some acids. Rhenium occurs naturally in molybdenum and copper ores and is often obtained as a byproduct of mining those elements. It exists in several compounds including fluorides, chlorides, bromides, iodides, oxides, sulfides, and tellurides. Rhenium has applications in high-temperature turbines and engines due to its strength and corrosion resistance at high temperatures.





![Compound
• Fluorides
• Rhenium hexafluoride: ReF6
• Rhenium tetrafluoride: ReF4
• Rhenium pentafluoride: ReF5
• Rhenium heptafluoride: ReF7
• Chlorides
• Rhenium hexachloride: ReCl6
• Rhenium tetrachloride: ReCl4
• Rhenium pentachloride: ReCl5
• Trirhenium nonachloride: [ReCl3]3
• Bromides
• Rhenium tetrabromide: ReBr4
• Rhenium pentabromide: ReBr5
• Trirhenium nonabromide: [ReBr3]3](https://image.slidesharecdn.com/rhenium-141117212104-conversion-gate01/75/Rhenium-6-2048.jpg)
![• Iodides
• Rhenium tetraiodide: ReI4
• Trirhenium nonaiodide: [ReI3]3
• Oxides
• Rhenium dioxide: ReO2
• Rhenium trioxide: ReO3
• Dirhenium trioxide: Re2O3
• Dirhenium heptoxide: Re2O7
• Sulfides
• Rhenium disulphide: ReS2
• Dirhenium heptasulphide: Re2S7
• Selenides
• none listed
• Tellurides
• Rhenium ditelluride: ReTe2](https://image.slidesharecdn.com/rhenium-141117212104-conversion-gate01/75/Rhenium-7-2048.jpg)