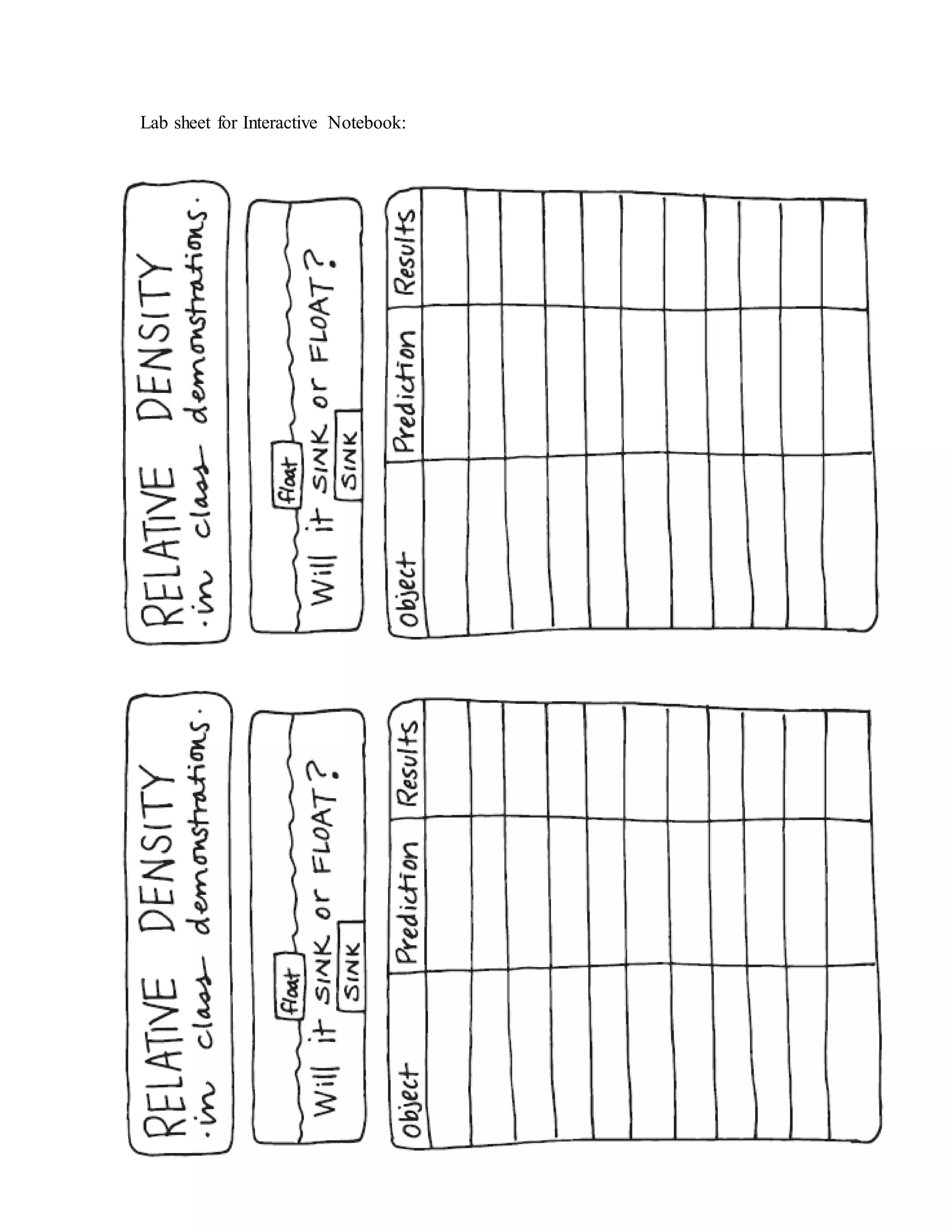
This document outlines a lesson plan on density and relative density for 5th grade students. The lesson objectives are for students to compare which objects sink or float in water and to predict and test the relative densities of liquids including salt water. Students will conduct experiments placing various objects in water and salt water to observe if they float or sink. They will make predictions and analyze the results to determine if the objects are more or less dense than water. Modifications for English language learners and special education students are provided, such as extended time, picture vocabulary, and lab assistants.