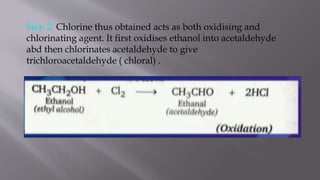
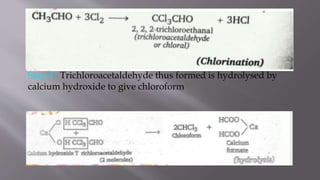
This document summarizes the preparation of chloroform from ethanol and bleaching powder. It describes the reaction steps: 1) bleaching powder produces chlorine when heated with water, 2) the chlorine oxidizes and chlorinates ethanol to form trichloroacetaldehyde, and 3) trichloroacetaldehyde is hydrolyzed by calcium hydroxide to produce chloroform. Some key properties of chloroform are provided, such as its colorless and heavy liquid nature with a sweet smell, boiling point of 334K, toxicity, and former use as an anesthetic.