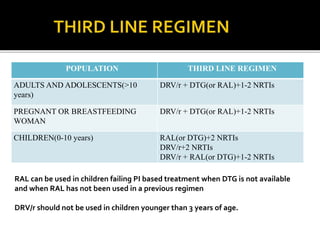
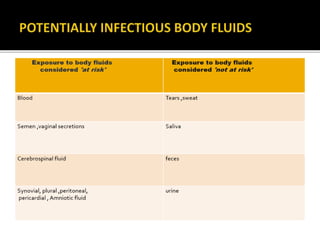
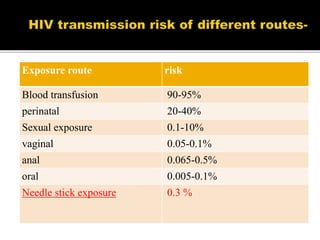
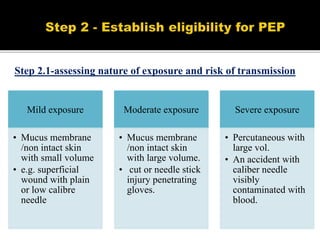
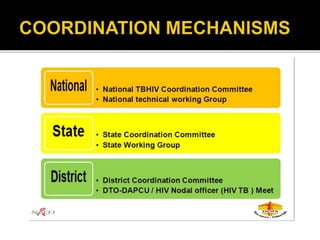
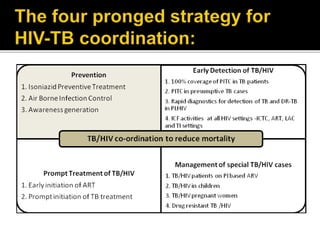
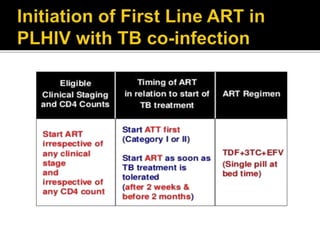
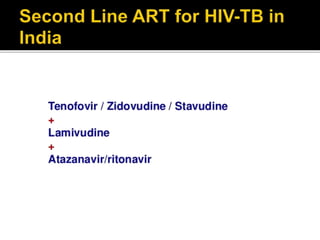
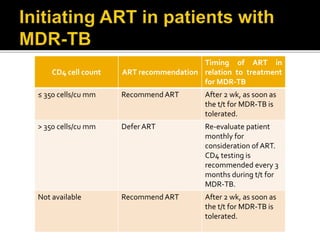
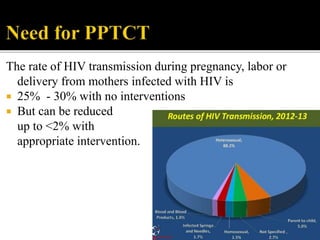
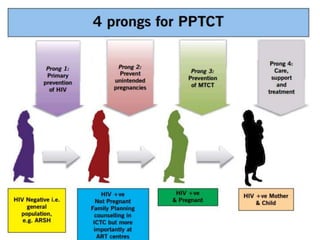
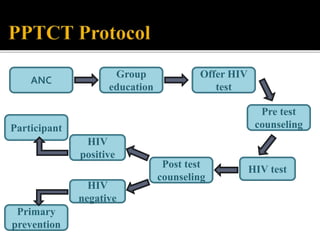
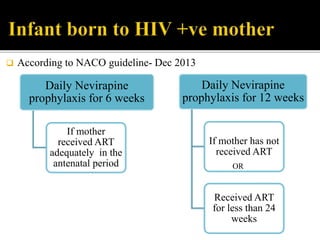
The document discusses India's significant HIV epidemic, highlighting statistics such as approximately 2.5 million people living with HIV and a 0.3% prevalence rate among adults in 2016. It details various HIV prevention and treatment strategies, including antiretroviral therapy (ART) guidelines, the importance of early detection through integrated counseling and testing centers (ICTCs), and post-exposure prophylaxis (PEP) protocols. Moreover, it emphasizes the need for accessible and sustainable healthcare services to improve the care, support, and treatment of individuals affected by HIV/AIDS.






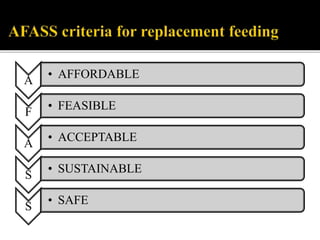





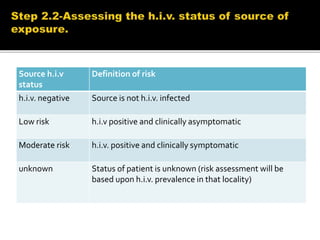
![FIRST LINE ART PREFFERED FIRST LINE
REGIMENS
ALTERNATIVE FIRST LINE
REGIMENS
ADULTS TDF+3TC[or FTC]+EFV AZT+3TC+EFV[OR NVP]
TDF+3TC[or FTC]+DTG
TDF+3TC[or FTC]+EFV
TDF+3TC[or FTC]+NVP
PREGNANT OR BREASTFEEDING
MOTHER
TDF+3TC[or FTC]+EFV AZT+3TC+EFV[or NVP]
TDF+3TC[or FTC]+NVP
ADOLESCENTS TDF+3TC[or FTC]+EFV AZT+3TC+EFV[or NVP]
TDF[or ABC]+3TC[or FTC]+DTG
TDF[or ABC]+3TC[or FTC]+EFV
TDF[or ABC]+3TC[or FTC]+NVP
CHILDREN 3 YEARS TO LESS
THAN 10 YEARS
ABC+3TC+EFV ABC+3TC+NVP
AZT+3TC+EFV[or NVP]
TDF+3TC[or FTC]+EFV[or NVP]
CHILDREN LESS THAN 3 YEARS ABC[or AZT]+3TC+LPV/r ABC[or AZT]+3TC+NVP](https://image.slidesharecdn.com/recentadvancesinaids-170625093009/85/Recent-advances-in-HIV-AIDS-24-320.jpg)
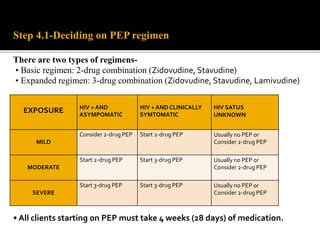
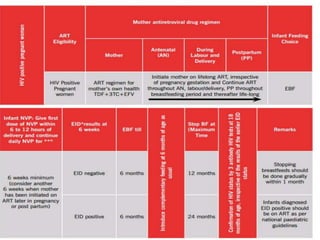
![PHASE OF HIV
MANAGEMENT
RECOMMENDED
HIV DIAGNOSIS HIV TESTING
CD4 CELL COUNT
TB SYMPTOMS SCREENING
FOLLOW UP
BEFORE ART
CD4 CELL COUNT[EVERY 6-12 MONTHS IF ART IS DELAYED]
RECEIVING ART HIV VIRAL LOAD[AT 6 MONTHS AND 12 MONTHS THEN EVERY 12
MONTHS THEREAFTER]
SUSPECTED
TREATMENT
FAILURE
SERUM CREATININE
PREGNANCY TEST](https://image.slidesharecdn.com/recentadvancesinaids-170625093009/85/Recent-advances-in-HIV-AIDS-25-320.jpg)
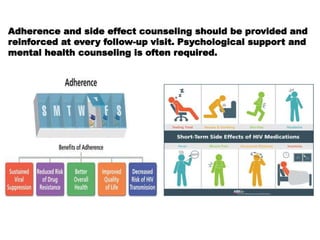
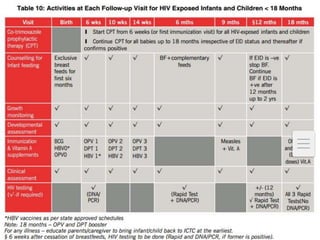
![FAILURE DEFINITION COMMENTS
CLINICAL NEW OR RECURRENT CLINICAL EVENT
[SEVERE IMMUNODEFICIENCY]AFTER 6
MONTHS
CONDITION MUST BE DIFFERENTIATED
FROM IRIS OCCURING AFTER INITIATING
ART
IMMUNOLOGICA
L
ADULTS & ADOLOSCENTS-
CD4 COUNT <250 CELLS/MM3
FOLLOWING CLINICAL FAILURE OR
PERSISTENT CD4 LEVELS BELOW 100
CELLS/MM3
CHILDREN-< 5 YEARS PERSISTENT CD4
LEVEL BELOW 200 CELLS/MM3
> 5 YEARS PERSISTENT CD4 LEVELS
BELOW 100CELLS/MM3
WITHOUT CONCOMITANT OR RECENT
INFECTION TO CAUSE A TRANSIENT
DECLINE IN THE CD4 CELL COUNT
VIROLOGICAL VIRAL LOAD ABOVE 1000 COPIES/ml
BASED ON TWO CONSECUTIVE VIRAL
LOAD MEASUREMENTS IN 3 MONTHS
WITH ADHERENCE SUPPORT FOLLOWING
THE FIRST VIRAL LOAD TEST
AN INDIVIDUAL MUST BE TAKING ART
FOR AT LEAST 6 MONTHS BEFORE IT CAN
BE DETERMINED THAT A REGIMEN HAS
FAILED](https://image.slidesharecdn.com/recentadvancesinaids-170625093009/85/Recent-advances-in-HIV-AIDS-26-320.jpg)
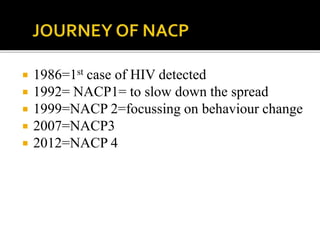
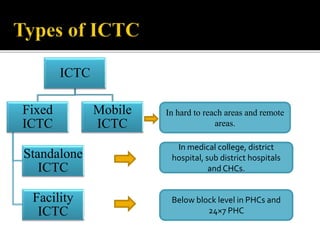
![POPULATION FAILING FIRST –LINE
REGIMEN
PREFERRED SECOND
LINE REGIMEN
ADULTS AND
ADOLESCENTS
2 NRTIs+EFV[ or NVP]
2 NRTIs +DTG
2NRTIs +ATV/r or LPV/r
PREGNANT OR BREAST
FEEDING
2NRTIs+EFV[or NVP] 2NRTIs+ATV/r or LPV/r
CHILDREN
<3 years
>3 years to <10 years
2NRTIs+LPV/r
2NRTIs+NVP
2NRTIs+LPV/r
2NRTIs+EFV[or NVP]
2NRTIs+RAL
2NRTIs+LPV/r
2NRTIs+EFV
2NRTIs +LPV/r
If ABC+3TC orTDF(or FTC) was used in first-failing regimen , AZT +3TC should
be used in second line](https://image.slidesharecdn.com/recentadvancesinaids-170625093009/85/Recent-advances-in-HIV-AIDS-27-320.jpg)