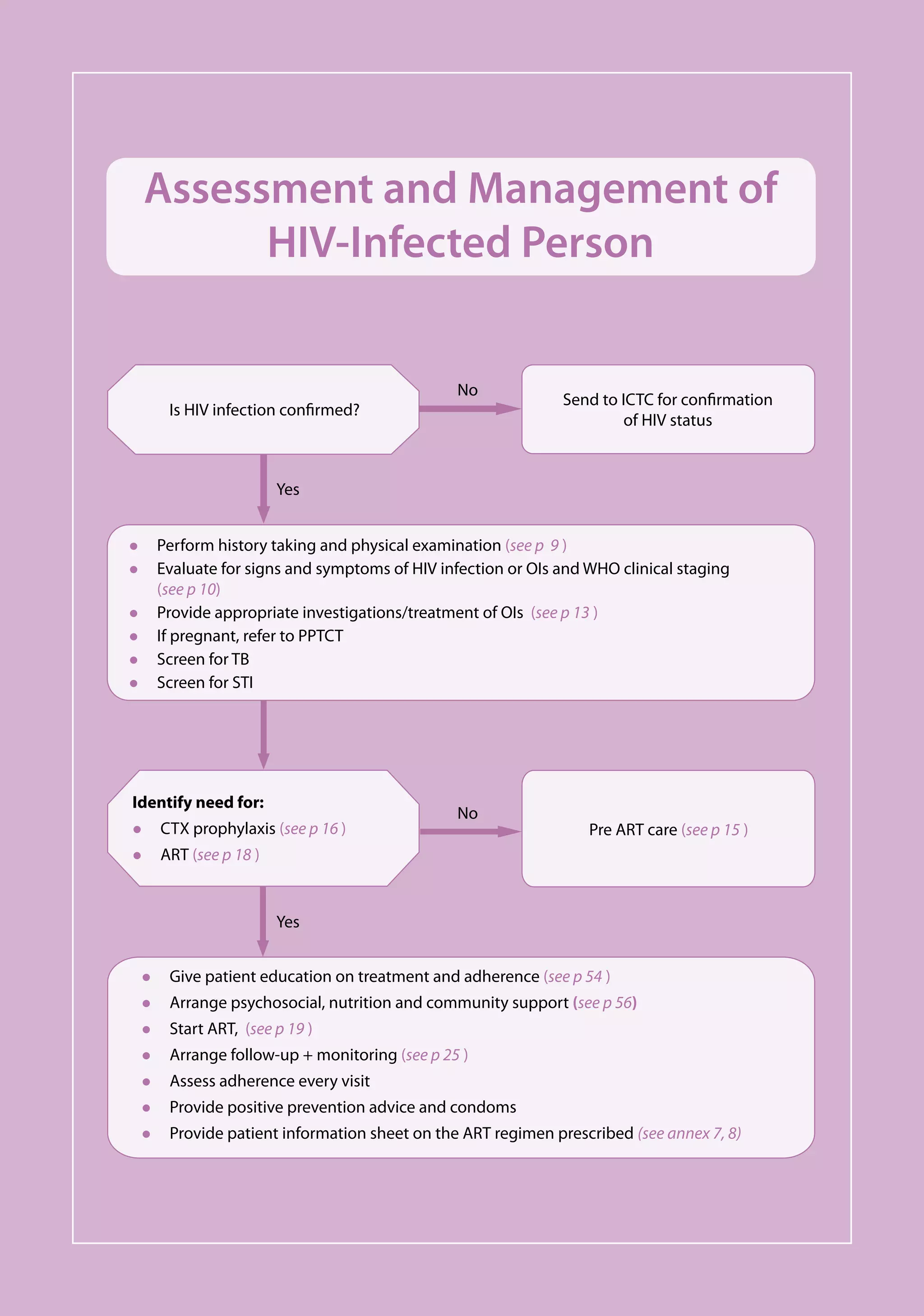
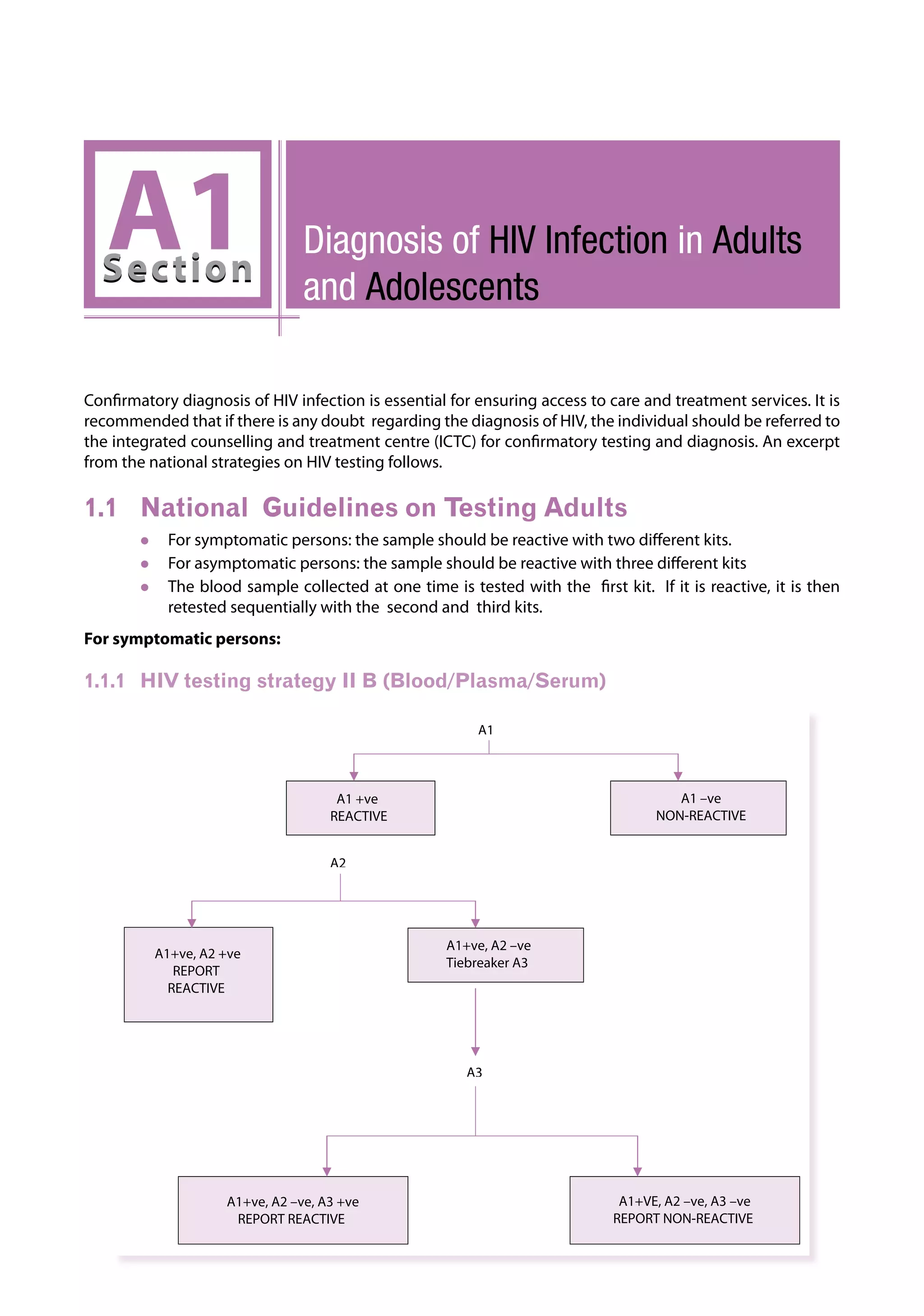
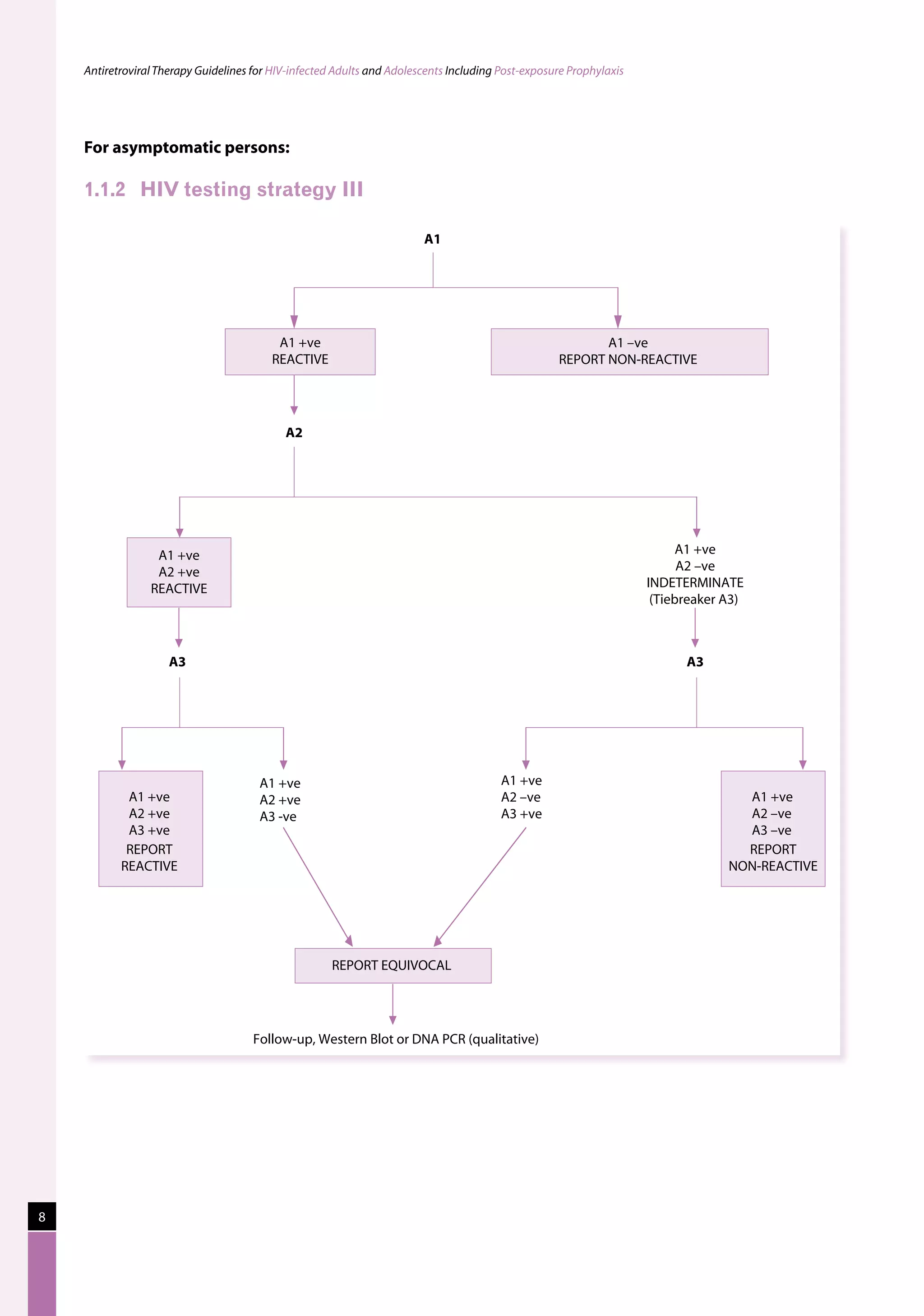
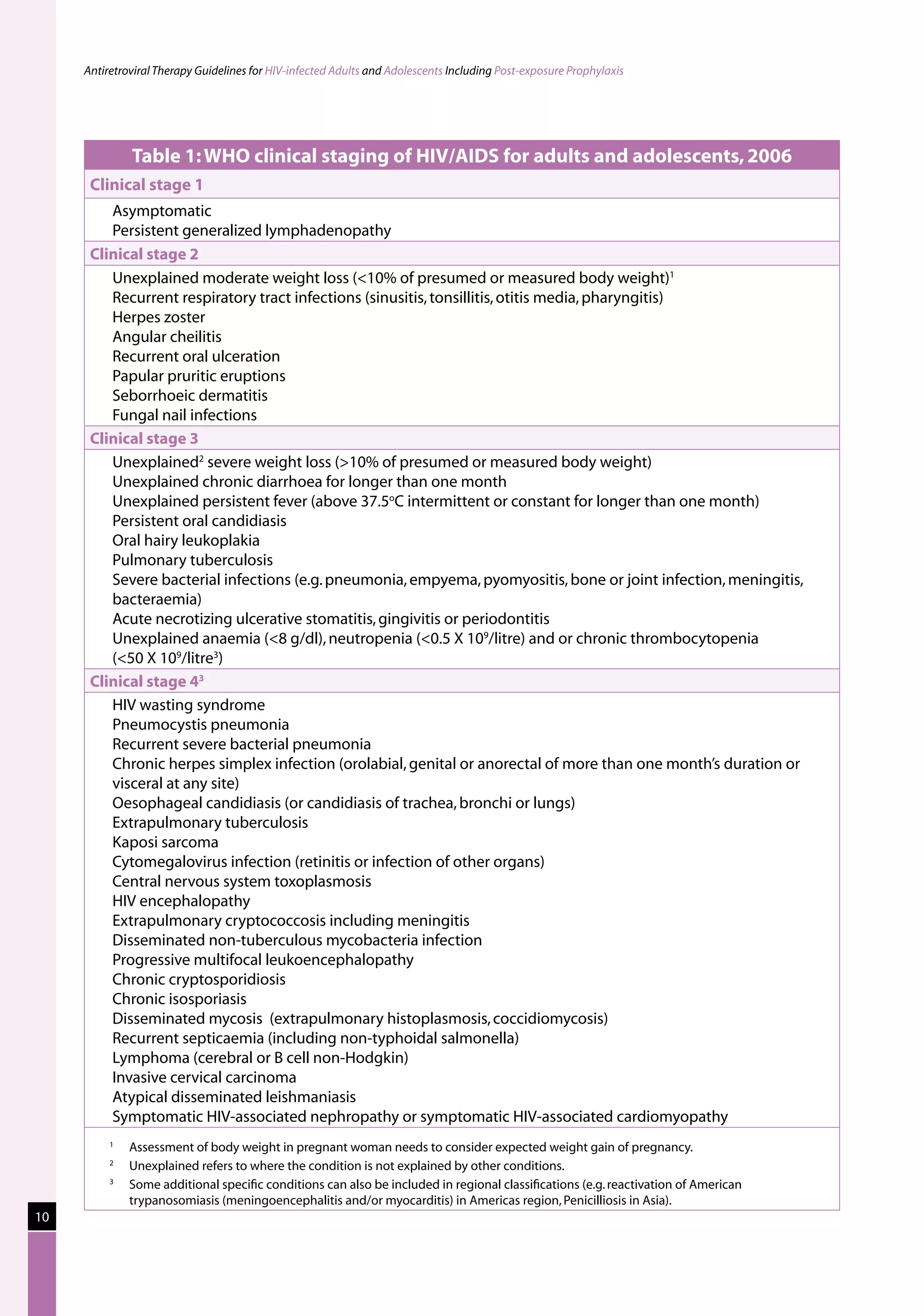
This document provides guidelines for antiretroviral therapy (ART) for HIV-infected adults and adolescents in India, including post-exposure prophylaxis.
The guidelines were developed by the National AIDS Control Organization of India (NACO) to assist physicians prescribing ART and to provide practical recommendations for treatment of HIV/AIDS in the context of India's national ART program and the role of the private sector.
The objectives of the guidelines are to provide long-term ART to eligible patients, monitor treatment outcomes, ensure high levels of individual drug adherence, increase life spans for those on ART, and enable patients to return to employment. ART is offered to all clinically eligible persons with HIV infection.




































![Antiretroviral Therapy Guidelines for HIV-infected Adults and Adolescents Including Post-exposure Prophylaxis
9.5 Substituting within a First-line Antiretroviral Drug
Regimen due to Drug Toxicity
A drug may need to be substituted to simplify the regimen so as to improve adherence, manage side-effects/
toxicities and/or reduce the cost of the regimen. The commonest example of substitution for avoiding long-
term toxicity is substituting from d4T and AZT to ABC or TDF.
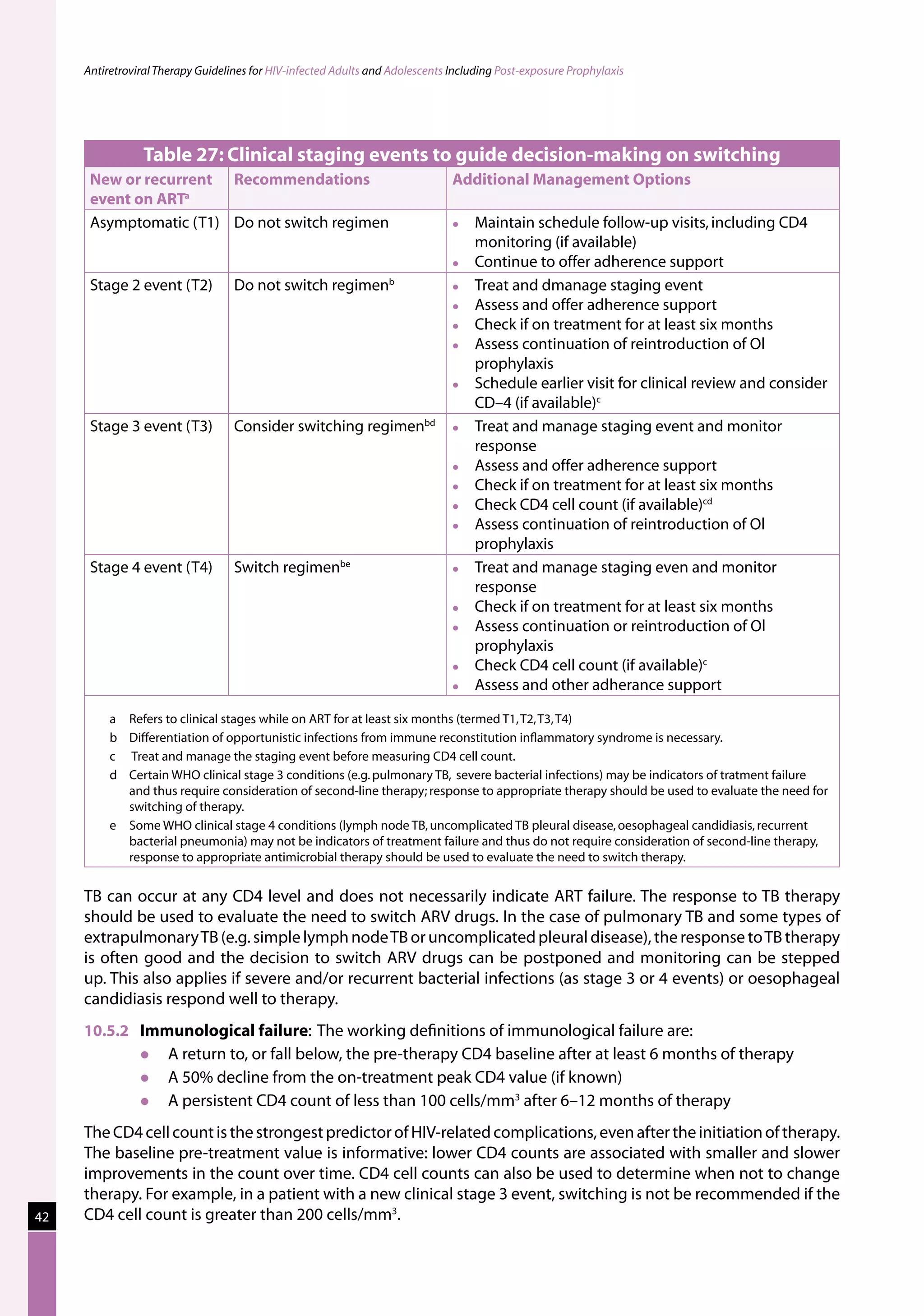
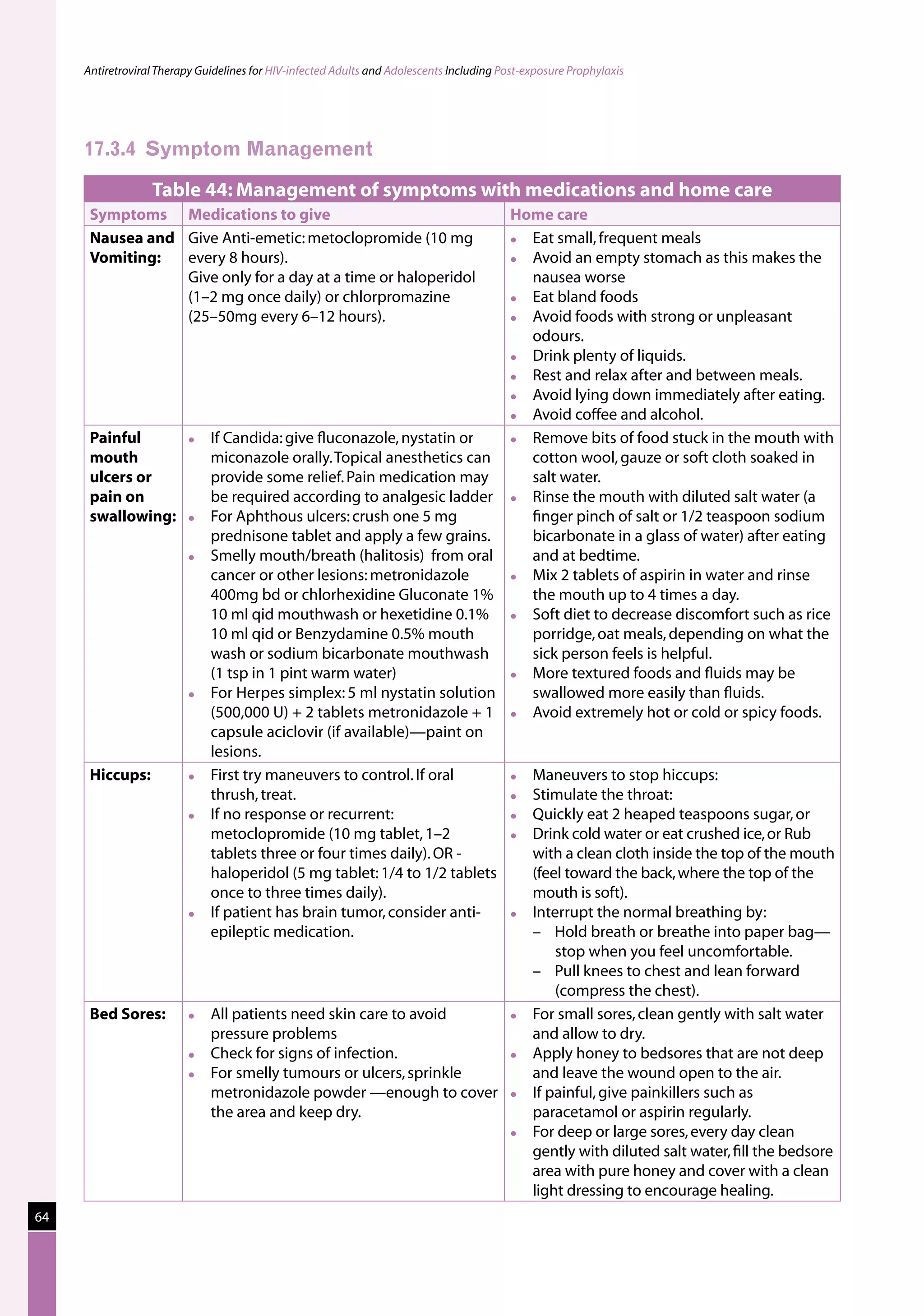
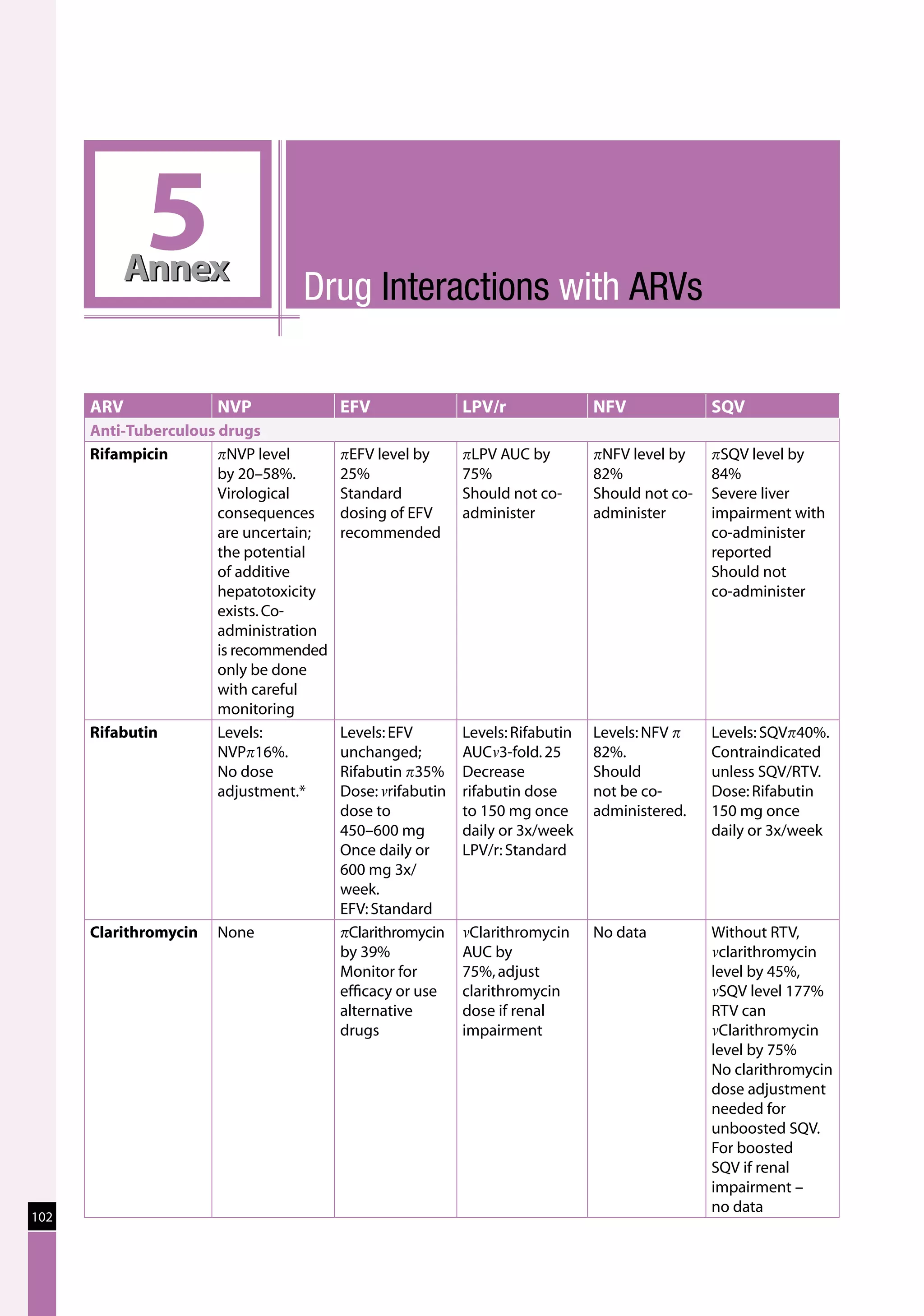
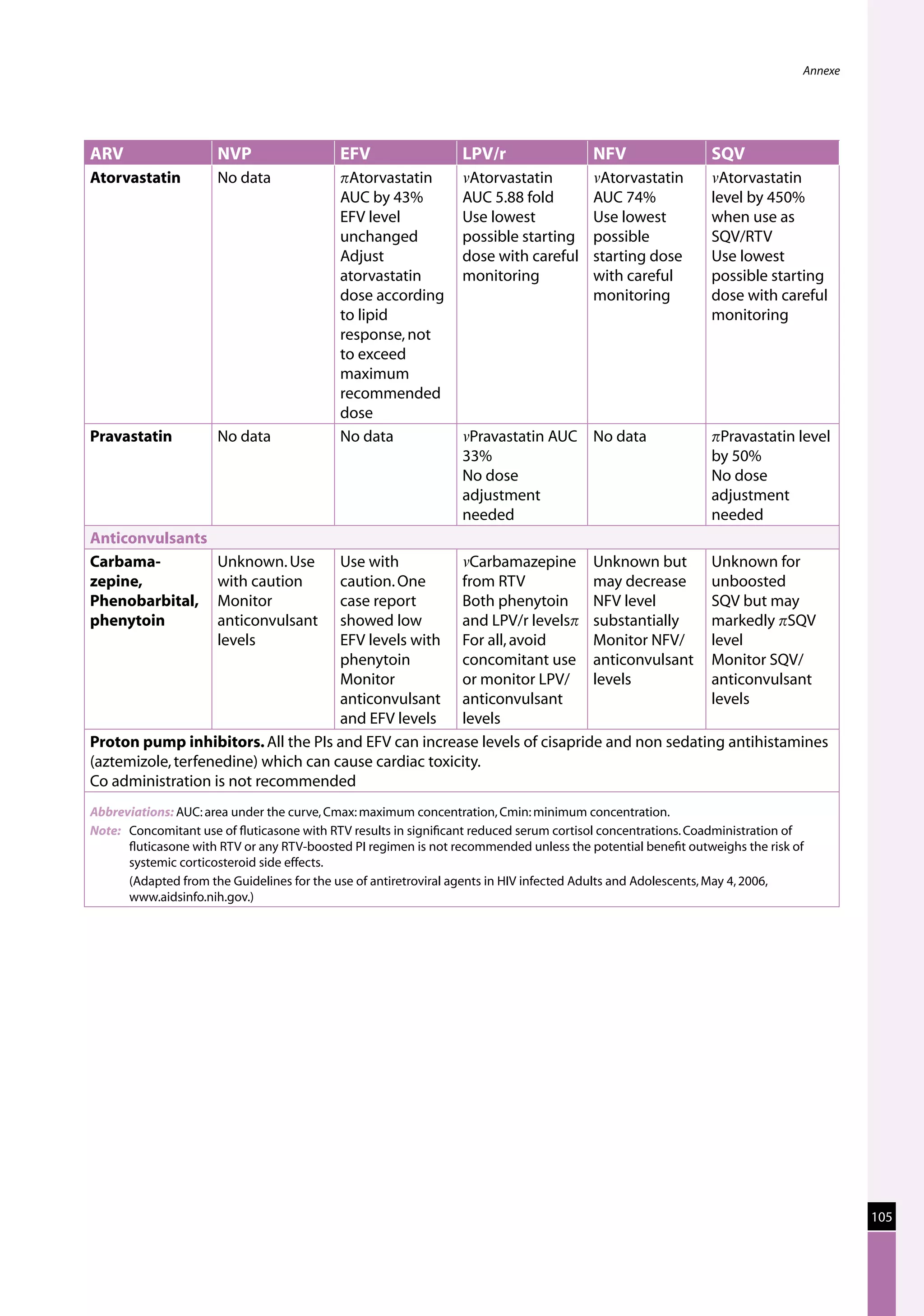
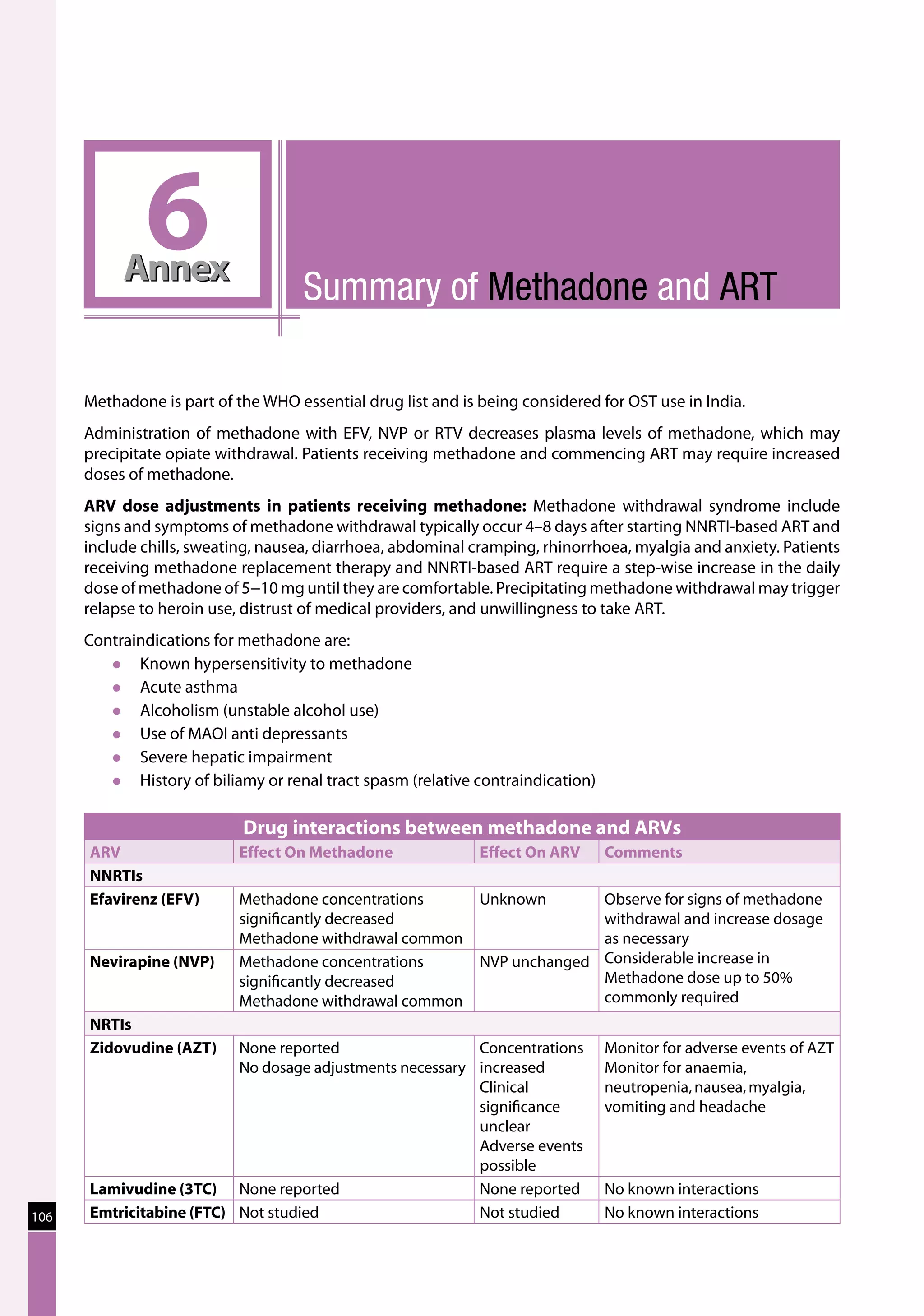
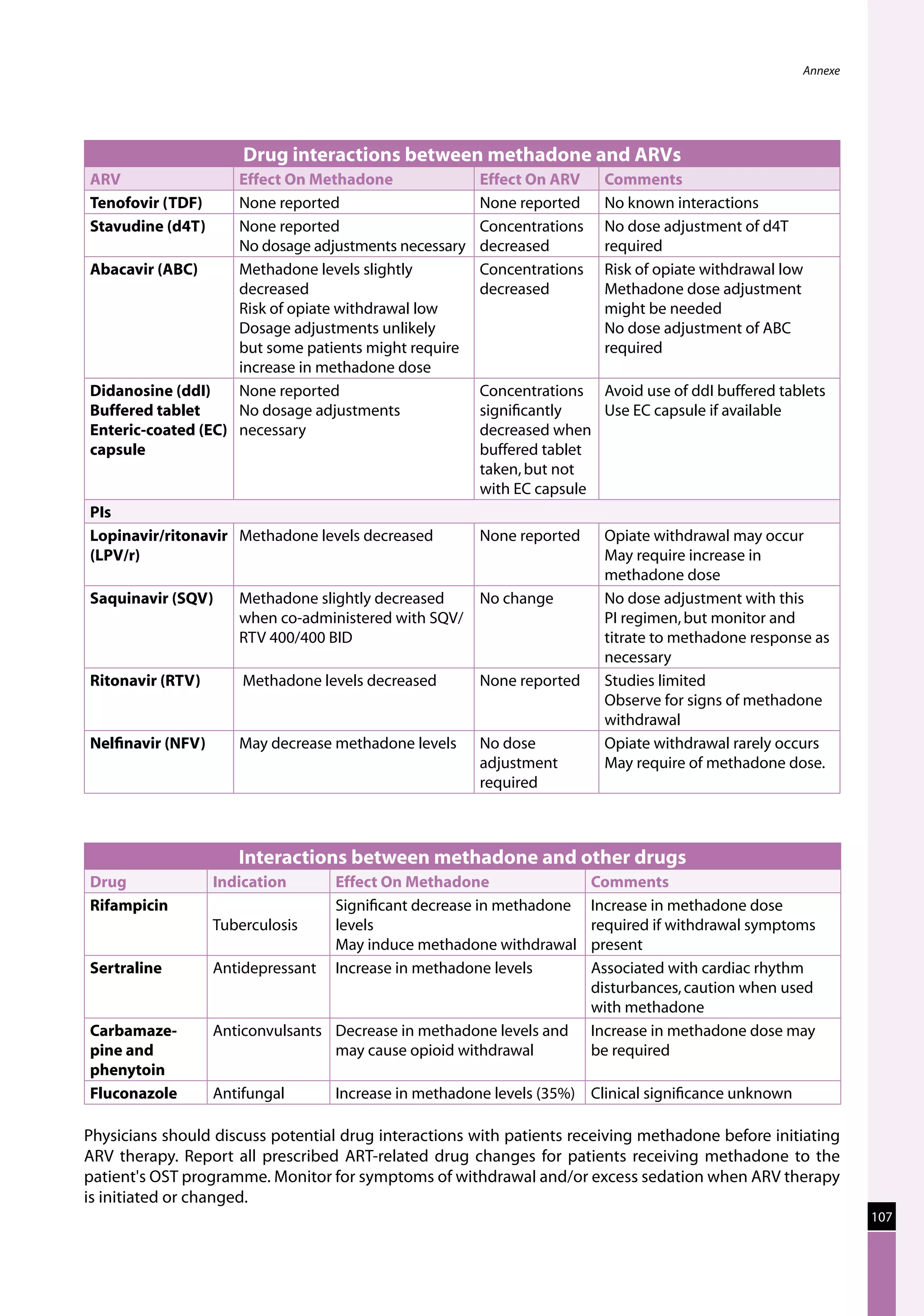
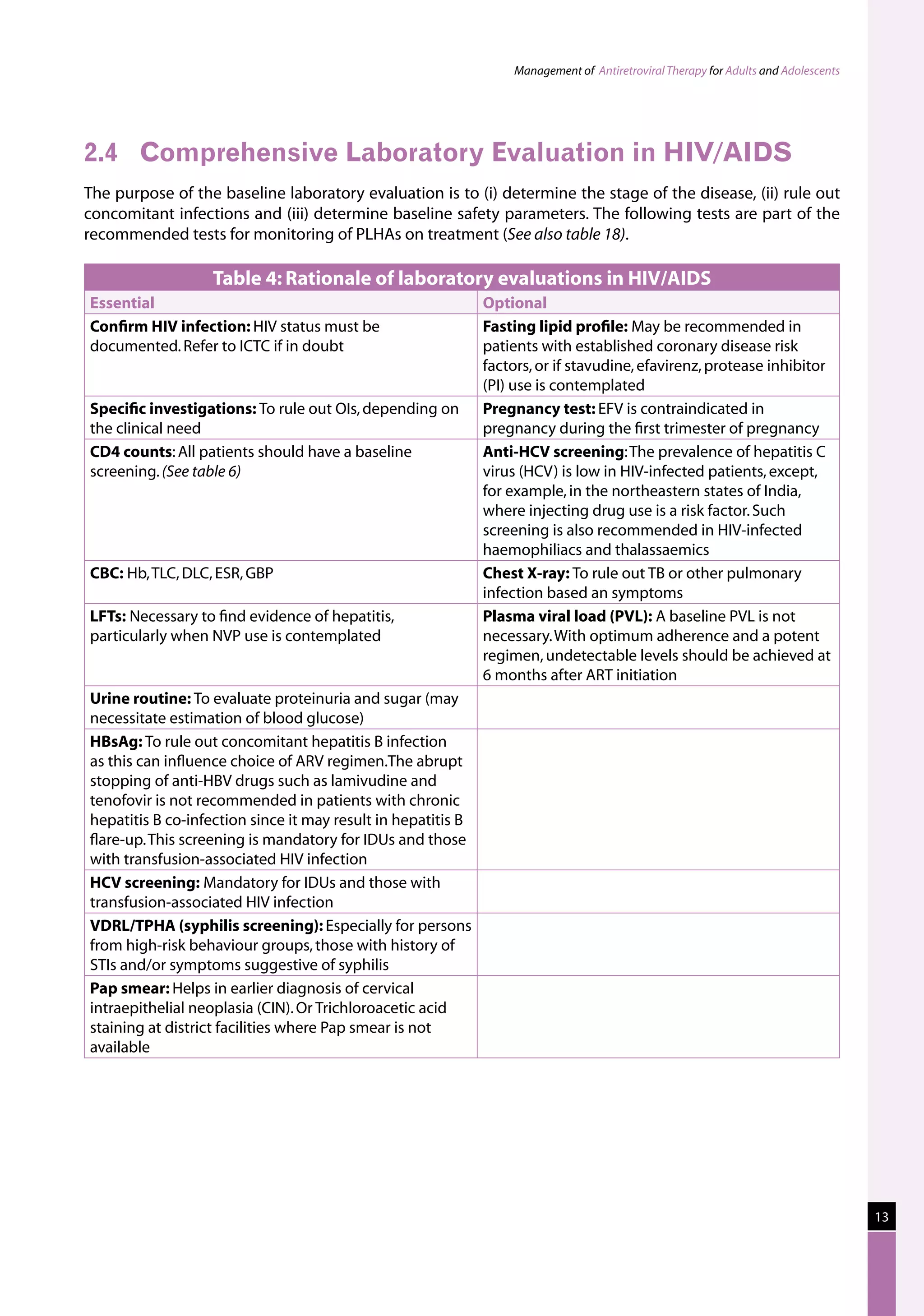
Table 23: Major toxicities caused by first-line ARV regimens and recommended
drug substitutions
Regimen Toxicity Drug substitution
D4T/3TC/NVP d4T-related neuropathy or pancreatitis Substitute with AZT
d4T-related lipoatrophy Substitute with TDF or ABC
(if available)
NVP-related severe hepatotoxicity Substitute with EFV
(except in first trimester of
pregnancy)
NVP-related severe rash (but not life-threatening) Substitute with EFV
NVP-related life-threatening rash (Stevens Johnson Substitute with PI or use
syndrome) “triple NRTI approach” which
reserves PI for second-line
treatment
AZT/3TC/NVP AZT-related persistent GI intolerance or severe Substitute with d4T
haematological toxicity
NVP-related severe hepatotoxicity Substitute with EFV (except
in pregnancy. In this
situation switch to NFV,
LPV/r or ABC.)
NVP-related severe rash (but not life-threatening) Substitute with EFV
NVP-related life-threatening rash (Stevens–Johnson Substitute with PI or use
syndrome) “triple NRTI approach” which
reserves PI for second-line
treatment
D4T/3TC/EFV d4T-related neuropathy or pancreatitis Substitute with AZT
d4T-related lipoatrophy Substitute with TDF or ABC
EFV-related persistent CNS toxicity Substitute with NVP
AZT/3TC/EFV AZT-related persistent GI intolerance or severe Substitute with d4T
haematological toxicity
EFV- related persistent CNS toxicity Substitute with NVP
Notes :
The general principle is that single-drug substitution for toxicity should be made within the same ARV class. [e.g. substitution of
d4T with AZT or TDF (for neuropathy), AZT with d4T or TDF (for anaemia), or EFV with NVP (for CNS toxicity or in pregnancy)].
Substituting d4T may not reverse lipodystrophy but may slow its progression. Besides AZT and TDF, ABC or ddI are acceptable
alternatives but are not available in the national programme.
If a life-threatening toxicity occurs, all ART should be stopped until the toxicity has resolved and a revised regimen commenced
when the patient has recovered.
38](https://image.slidesharecdn.com/nacoguidelinesforhiv-aidsmanagement-120602005348-phpapp01/75/Naco-guidelines-for-hiv-aids-management-46-2048.jpg)