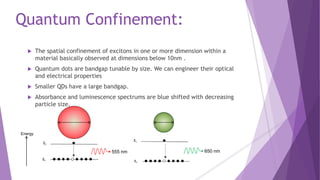
Quantum dots are nanoscale semiconductor crystals that exhibit quantum confinement effects, influencing their optical and electrical properties. They can be tuned by size and have a range of applications, including light emitters, biological imaging, and solar cells. Their unique properties make them useful in various fields, such as quantum computation and medicine.