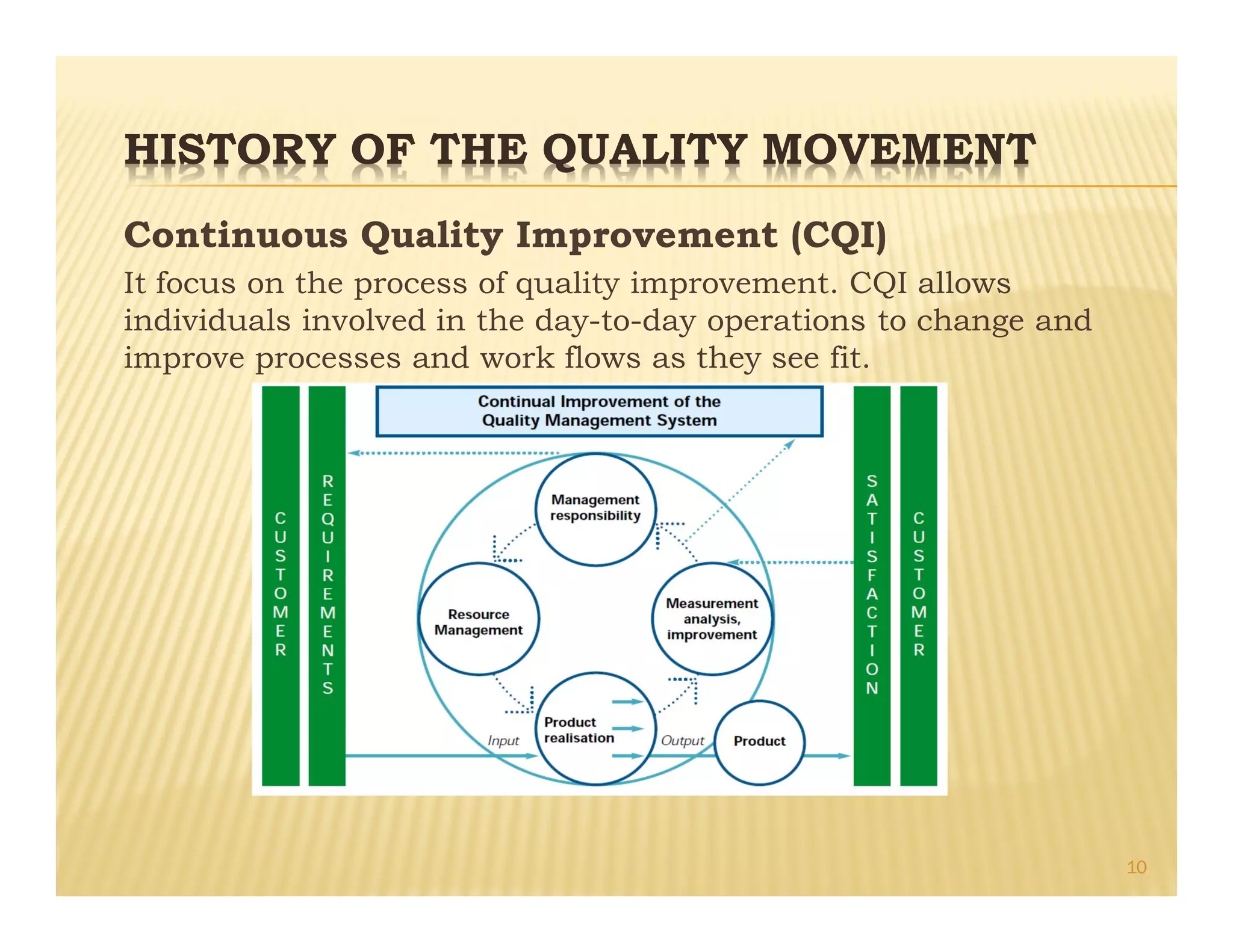
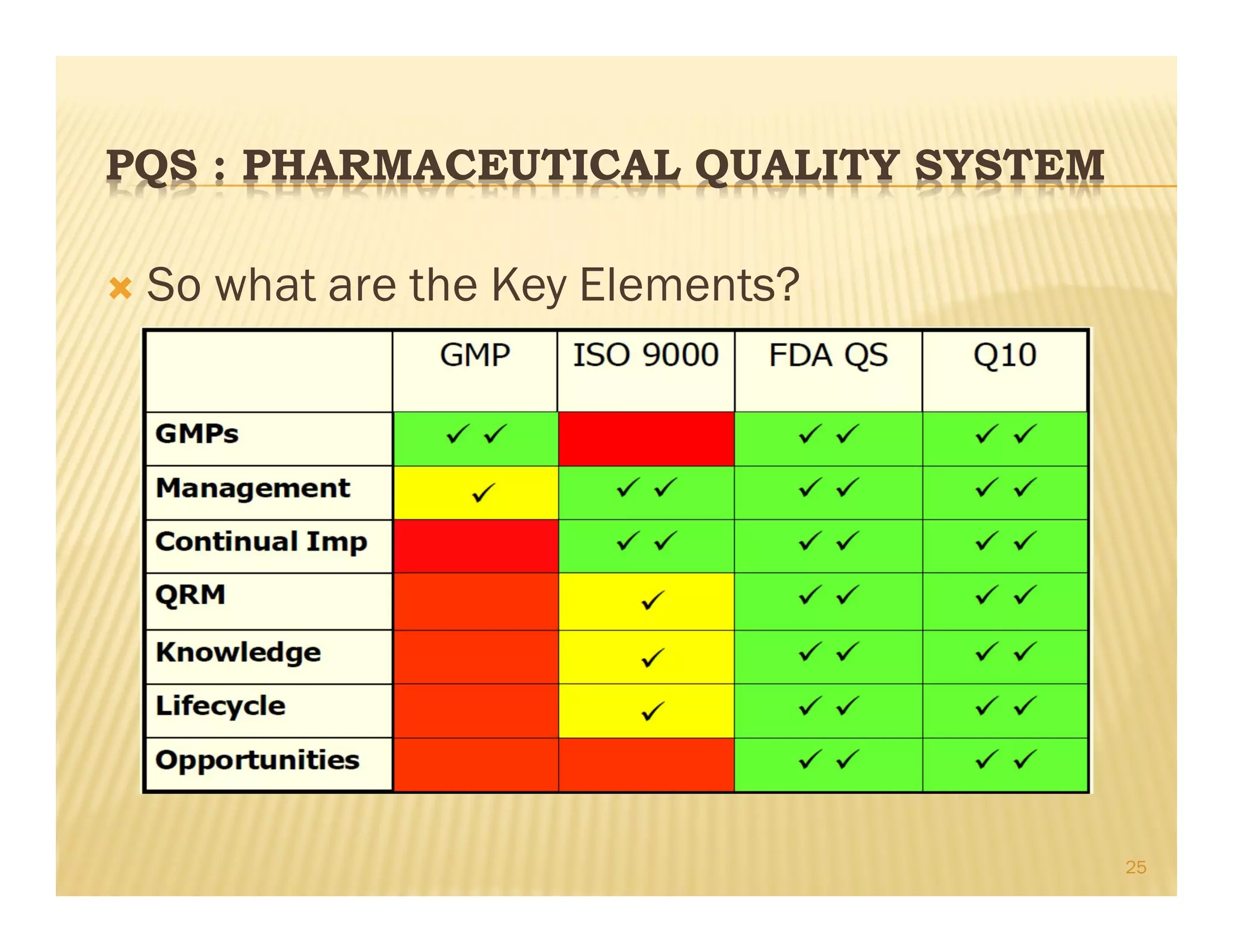
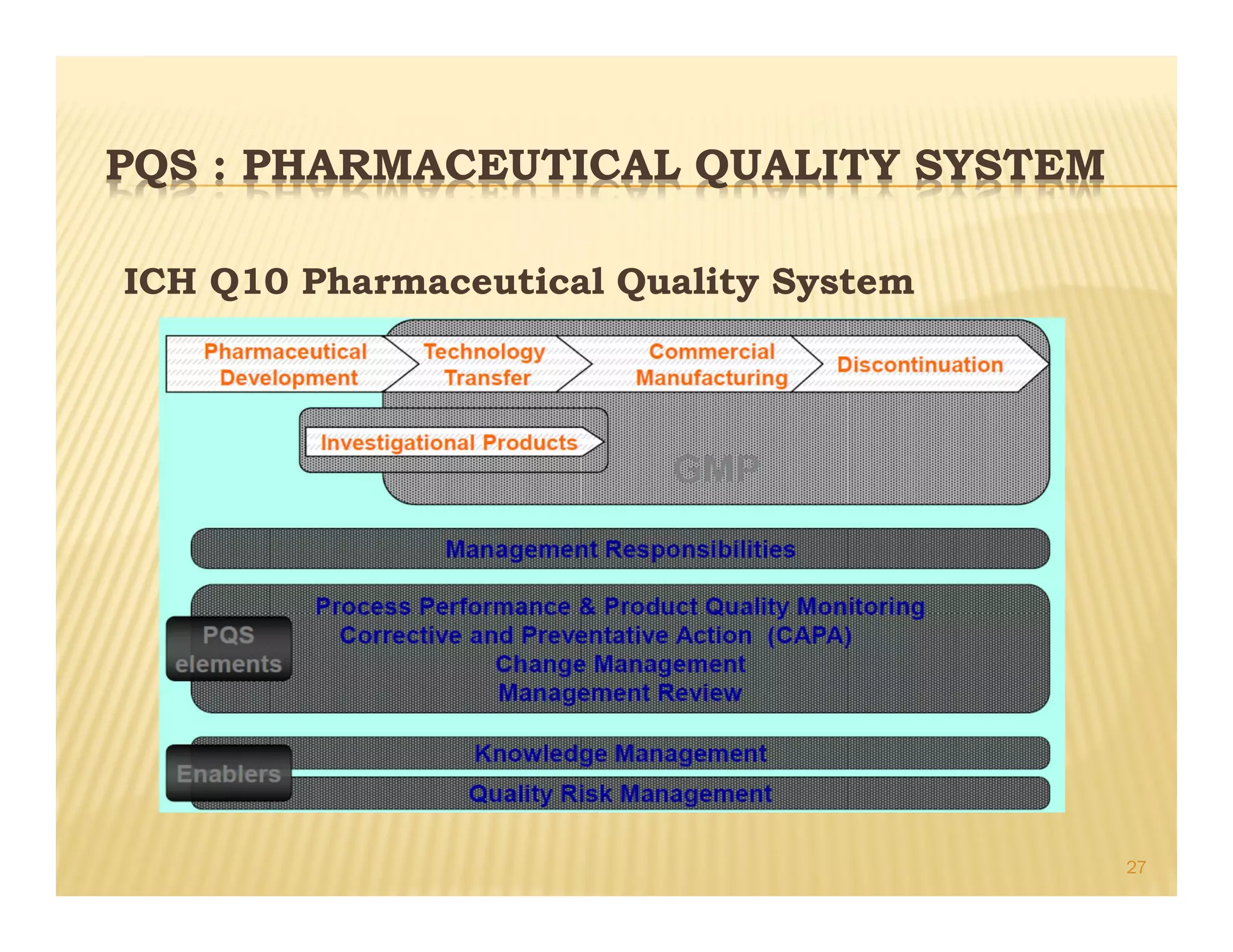
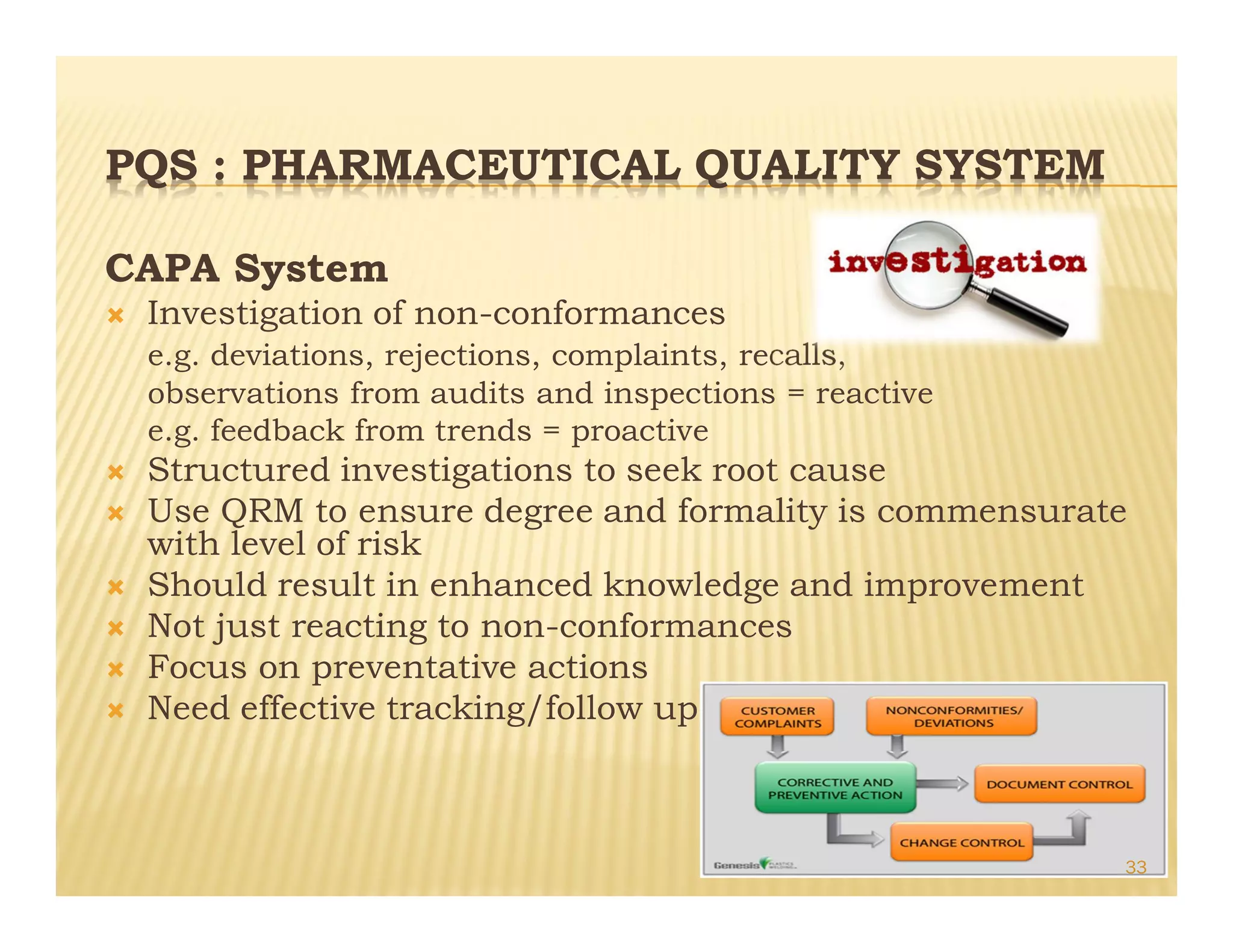
This document provides an overview of quality management systems and their history. It defines quality based on customer perceptions and needs. Quality management systems allow organizations to meet quality levels, consumer requirements, and technology changes. The document traces the development of quality management from early thinkers like Deming and Juran who helped Japanese companies, to the growth of approaches like total quality management, ISO standards, six sigma at Motorola, and continuous quality improvement. It outlines eight quality management principles and discusses pharmaceutical quality systems and ICH Q10, which promote a lifecycle approach to quality over compliance.