This document discusses quality and formulation of medicines. It covers several topics:
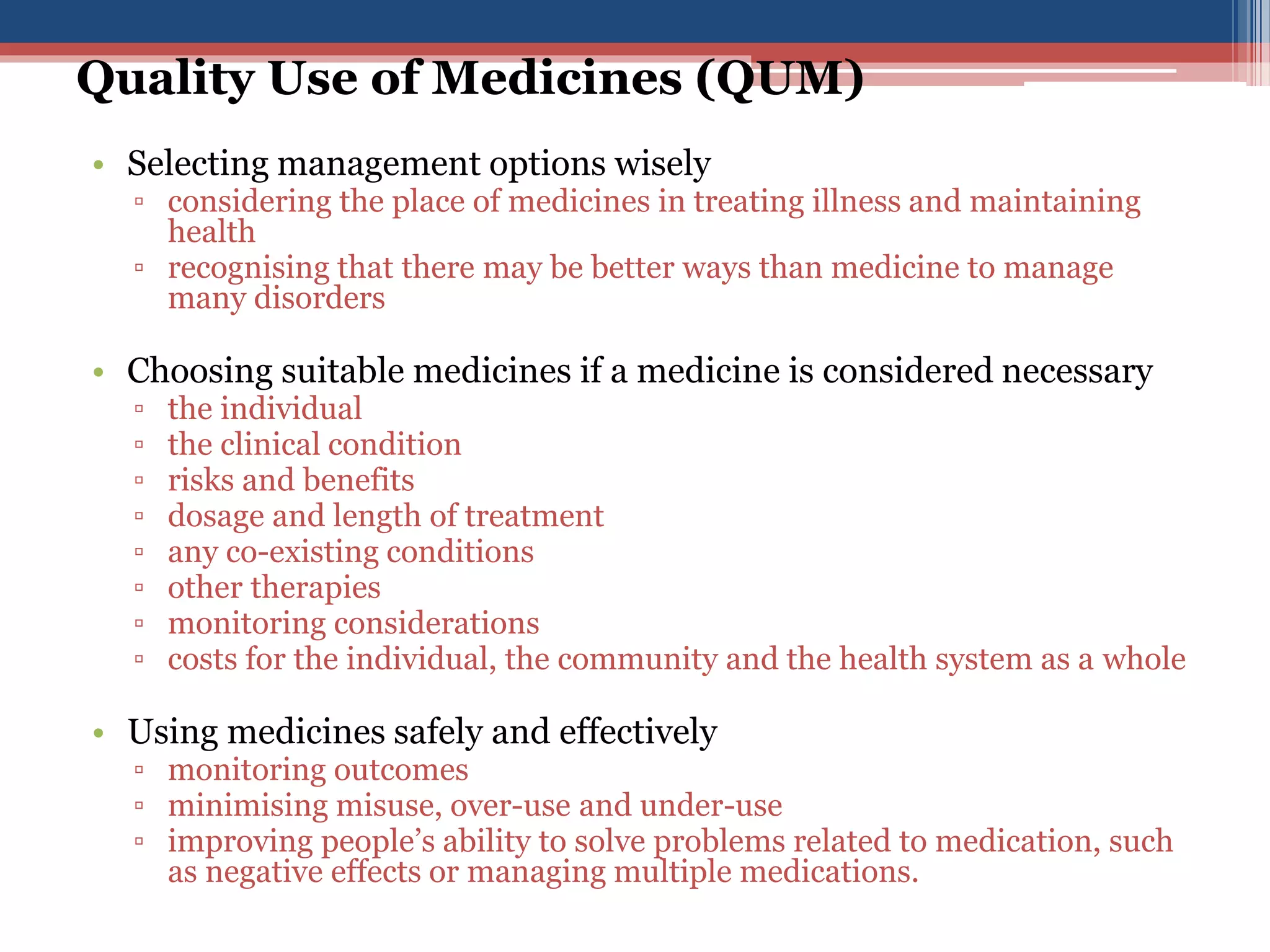
1) Quality use of medicines includes selecting management options wisely based on the individual, condition, risks/benefits, and costs. It also involves using medicines safely and effectively.
2) Manufacturing of pharmaceutical products must follow good manufacturing practices to ensure quality, including production and quality control procedures.
3) Non-clinical safety studies are conducted on active substances and formulations to explore safety and inform clinical trials. Safety testing assesses margins of safety.