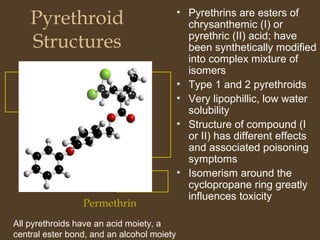
Pyrethroids are synthetic pesticides derived from natural pyrethrins produced by chrysanthemum flowers. They are designed to be more toxic to insects and less degradable than pyrethrins. Pyrethroids come in Type I and Type II variants, with Type II having enhanced toxicity due to cyano groups. They are absorbed through ingestion, inhalation, and dermal contact. Pyrethroids act by altering sodium and chloride channel function in nerves, causing hyperexcitability. They are metabolized in the liver but their lipophilicity allows accumulation in fat tissues. Their mechanism of toxicity involves prolonged nerve and muscle stimulation.