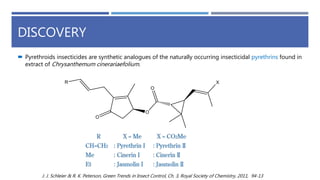
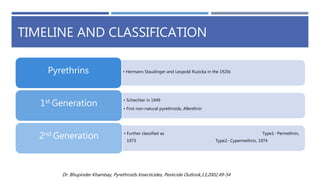
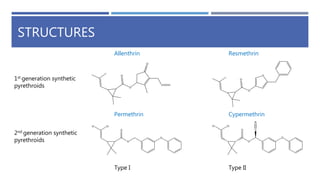
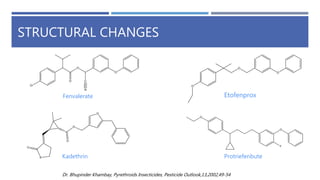
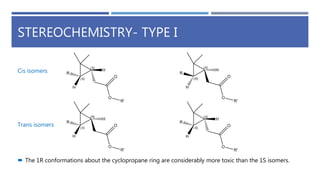
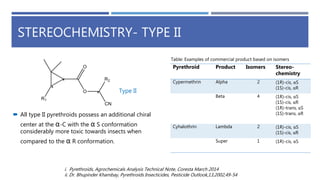
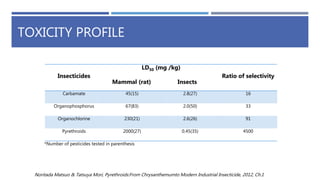
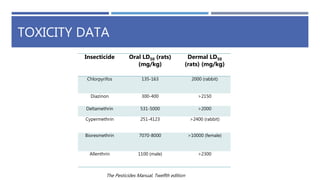
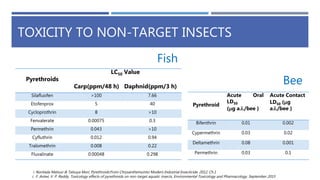
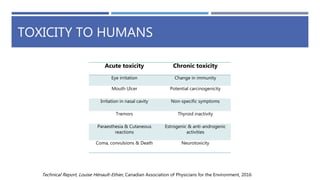
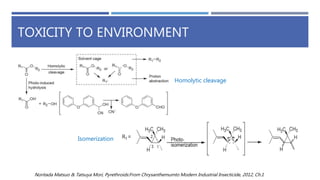
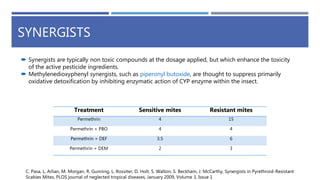
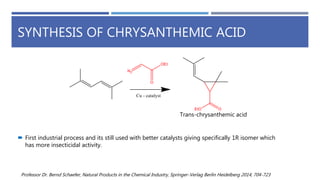
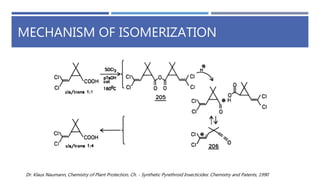
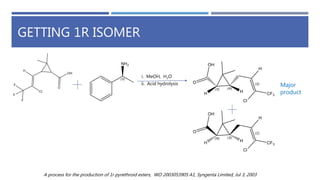
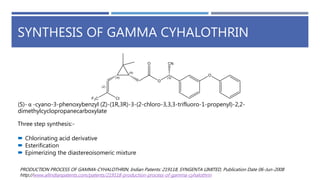
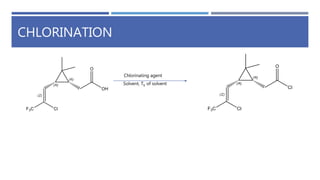
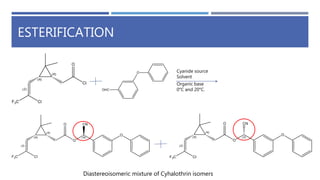
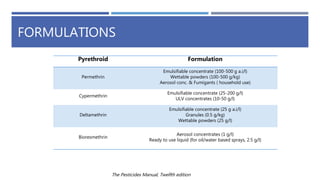
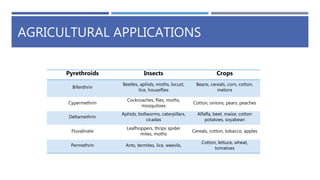
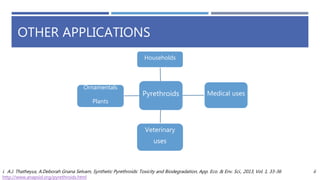
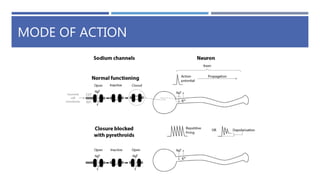
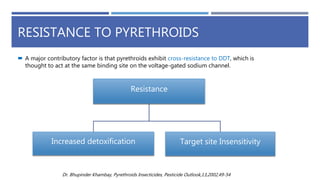
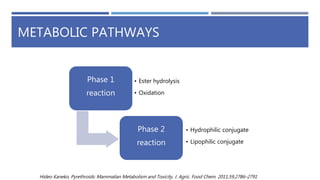
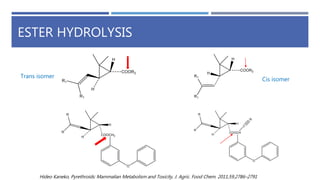
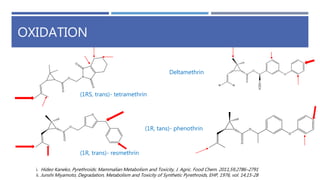
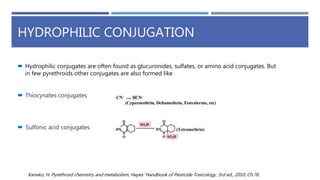
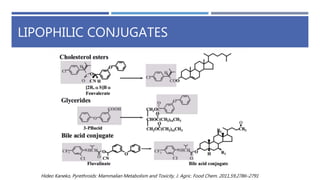
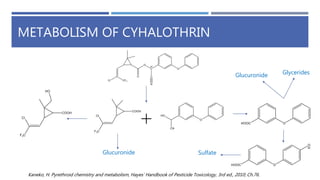
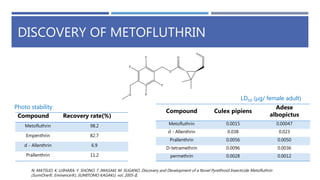
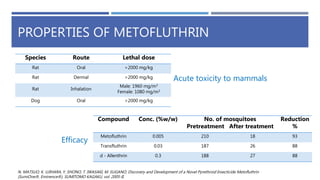
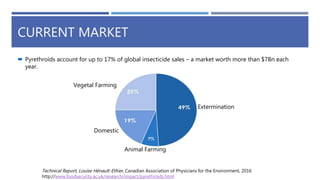
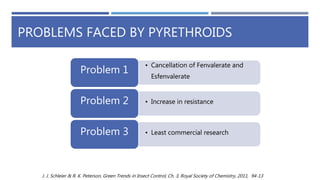
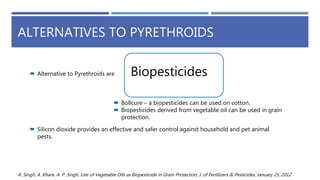
The document is a seminar by Meet Patel detailing synthetic pyrethroids, which are synthetic insecticides derived from pyrethrins, including their discovery, classifications, modes of action, and toxicity profiles. It discusses various types of synthetic pyrethroids, their synthesis processes, applications in agriculture and medical uses, and their effects on target and non-target organisms. Additionally, it highlights recent developments in synthetic pyrethroids and their metabolic mechanisms.