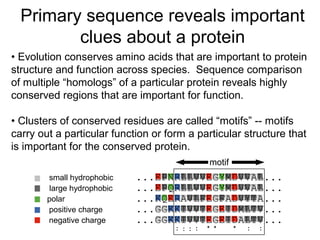
This document provides an overview of protein structure from primary to quaternary levels. It discusses the building blocks of proteins including amino acids and peptide bonds. Secondary structures like alpha helices and beta sheets are explained. Tertiary structure refers to the global folding of the protein chain. Quaternary structure involves the assembly of multiple protein subunits. Examples are given of protein complexes demonstrating tertiary and quaternary levels of structure. The document also outlines different classes of proteins based on function, structure, and cellular localization.