The document provides an overview of protein metabolism. It discusses the key topics of:
- Protein structure and functions in the body.
- The amino acid pool and how tissues draw from and contribute to it.
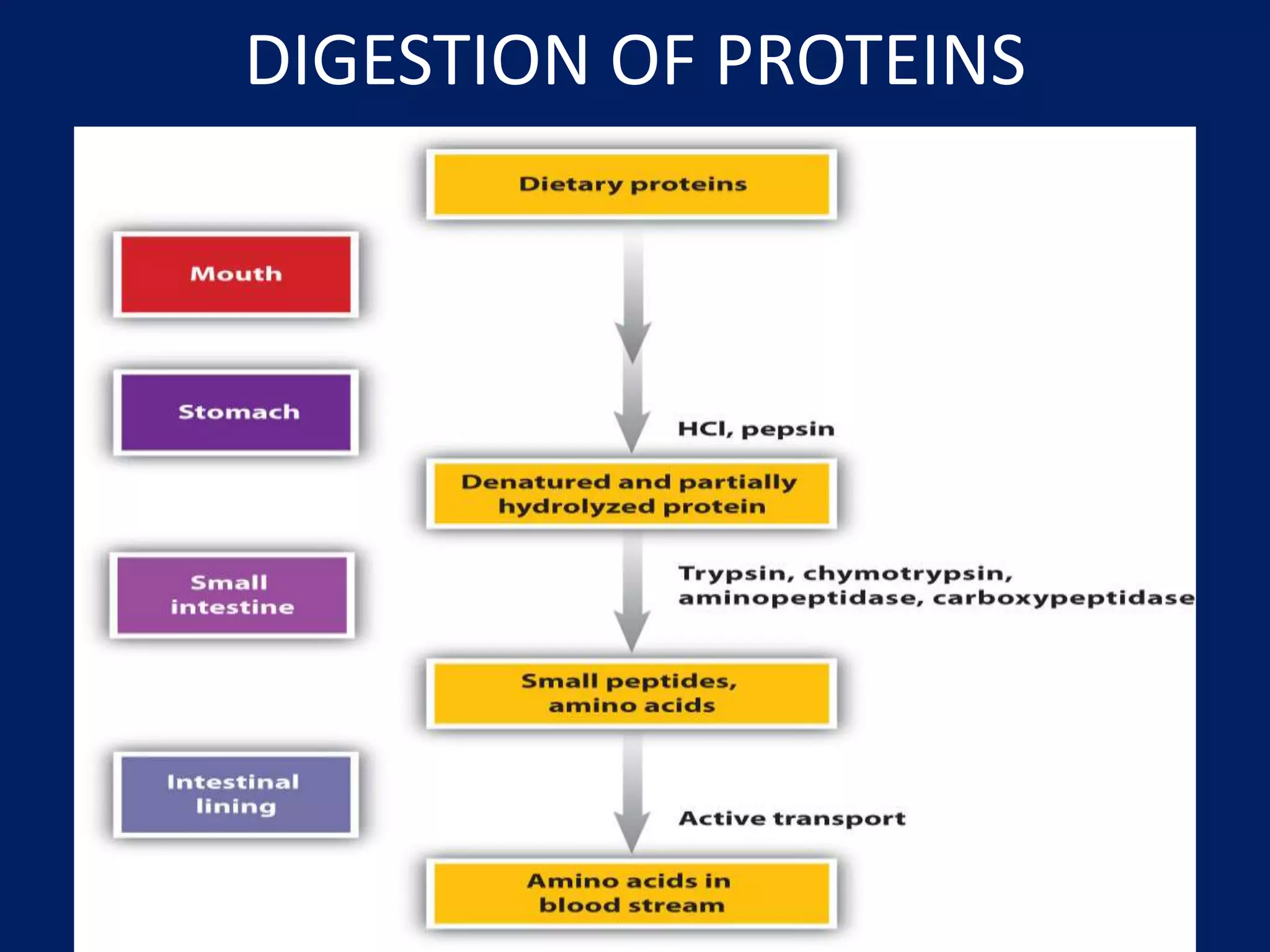
- The digestion of proteins in the body.
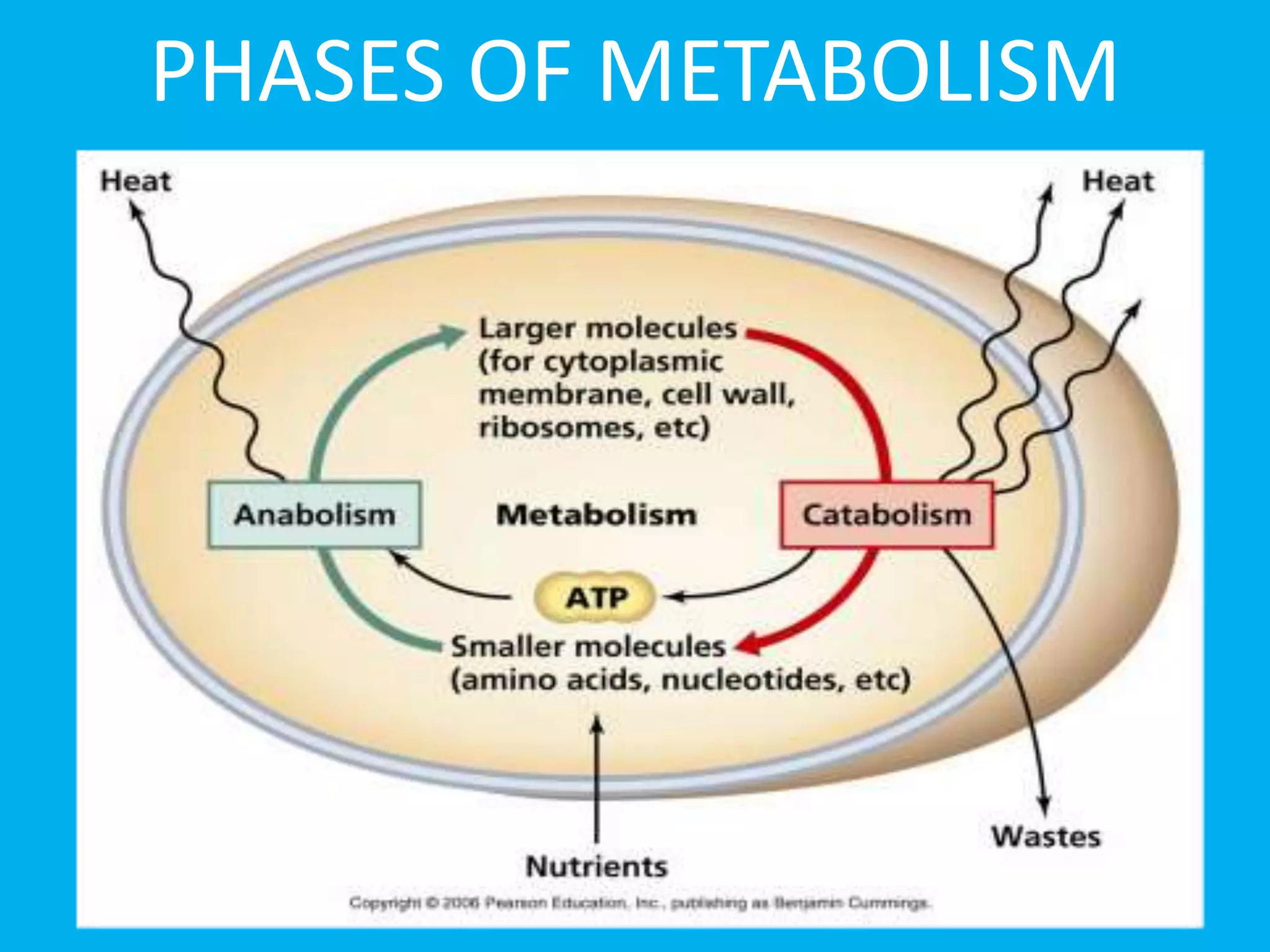
- The two phases of protein metabolism - anabolism and catabolism.
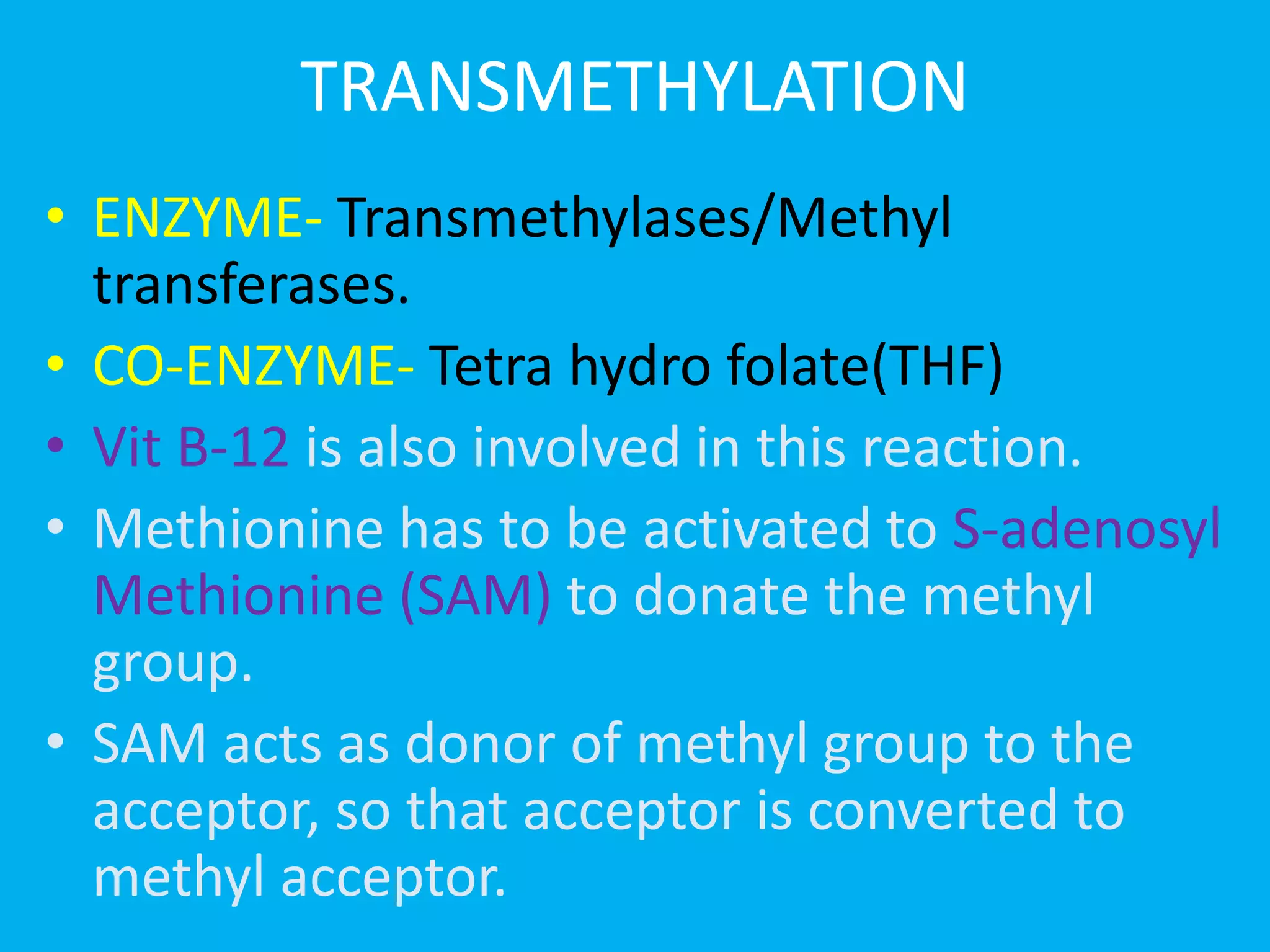
- The major catabolic pathways in the liver that break down amino acids including deamination, transamination, decarboxylation, and transmethylation.
- The ornithine or urea cycle, which occurs primarily in the liver and converts ammonia into urea for excretion from the body.