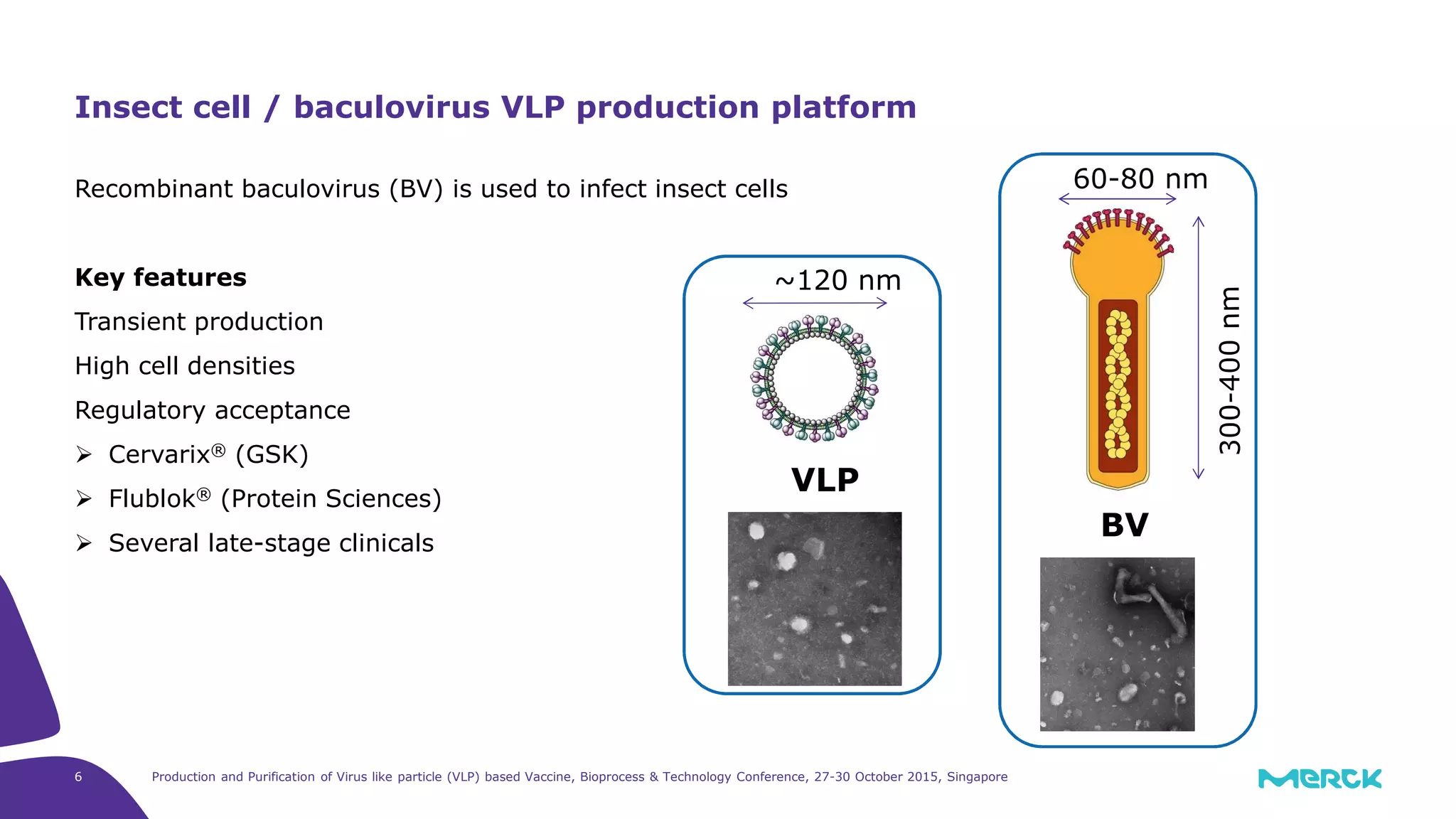
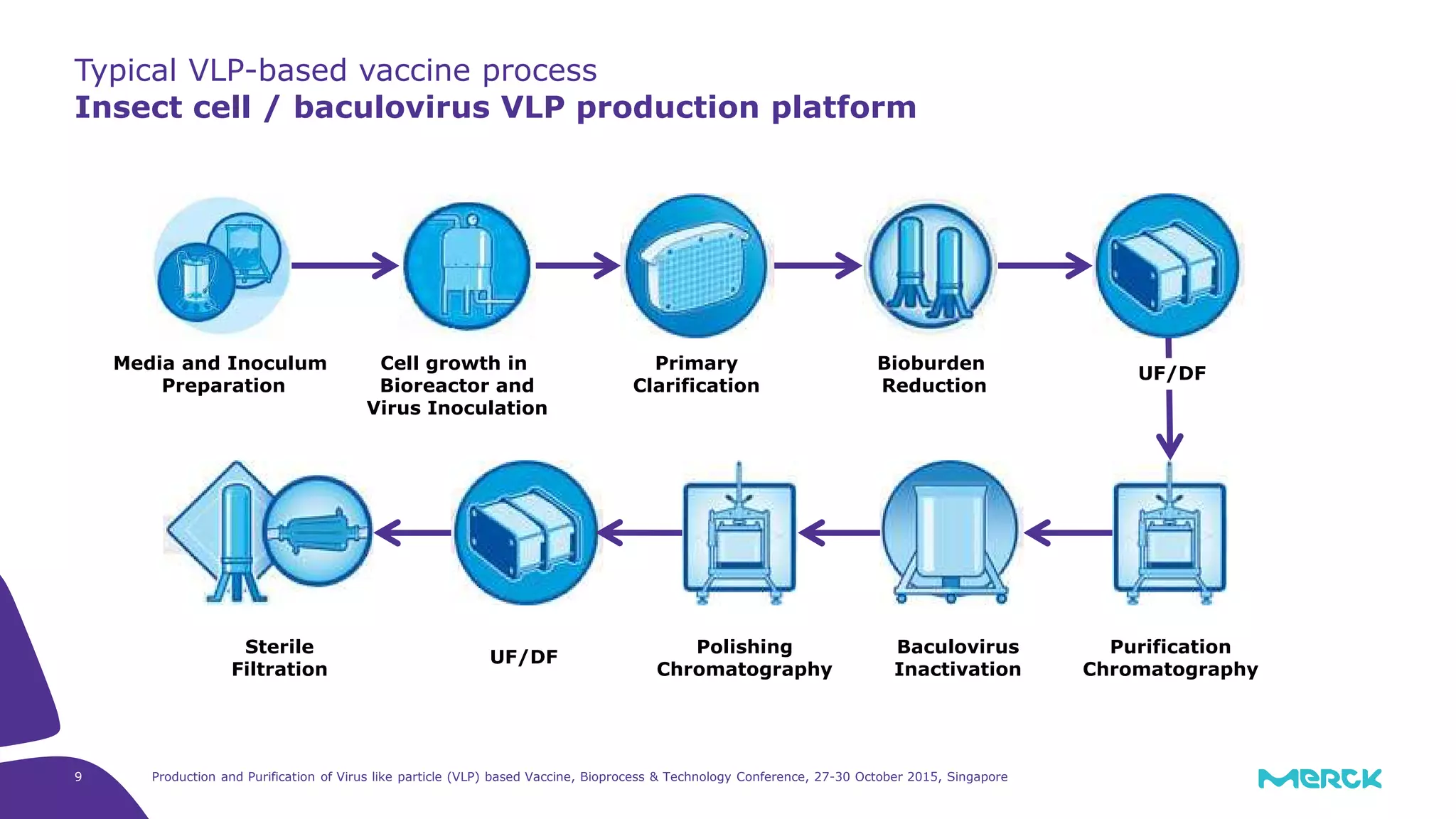
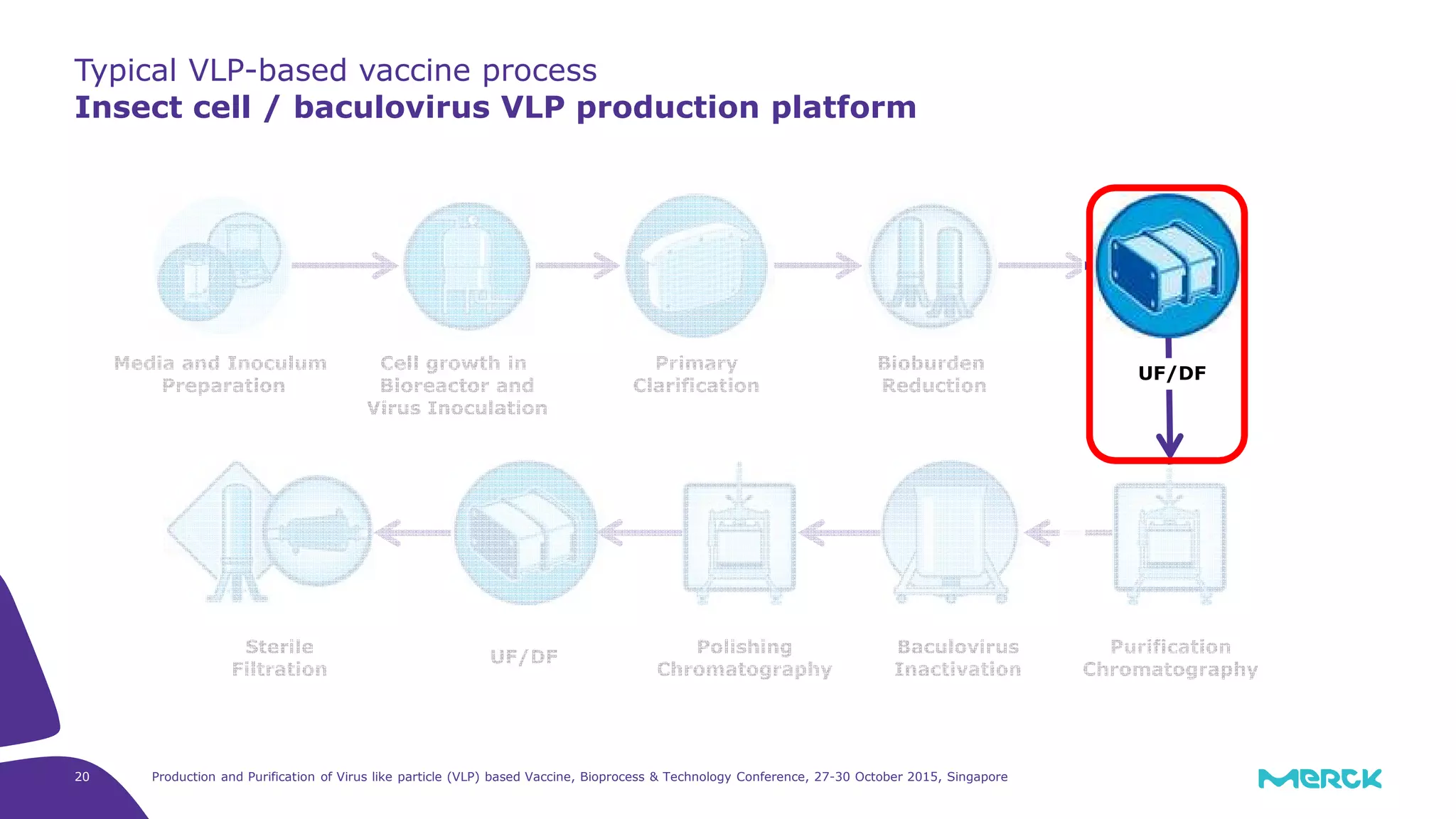
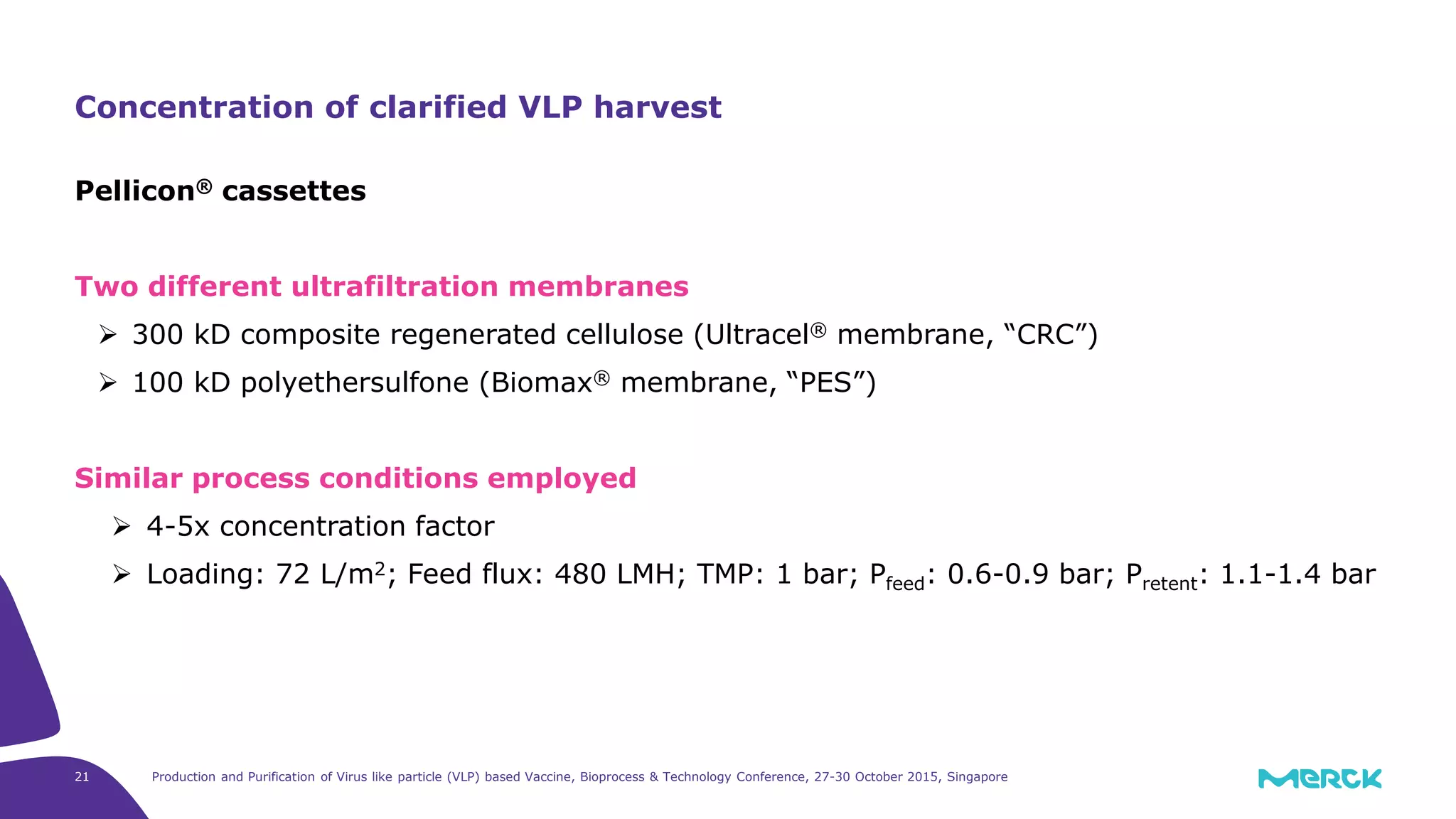
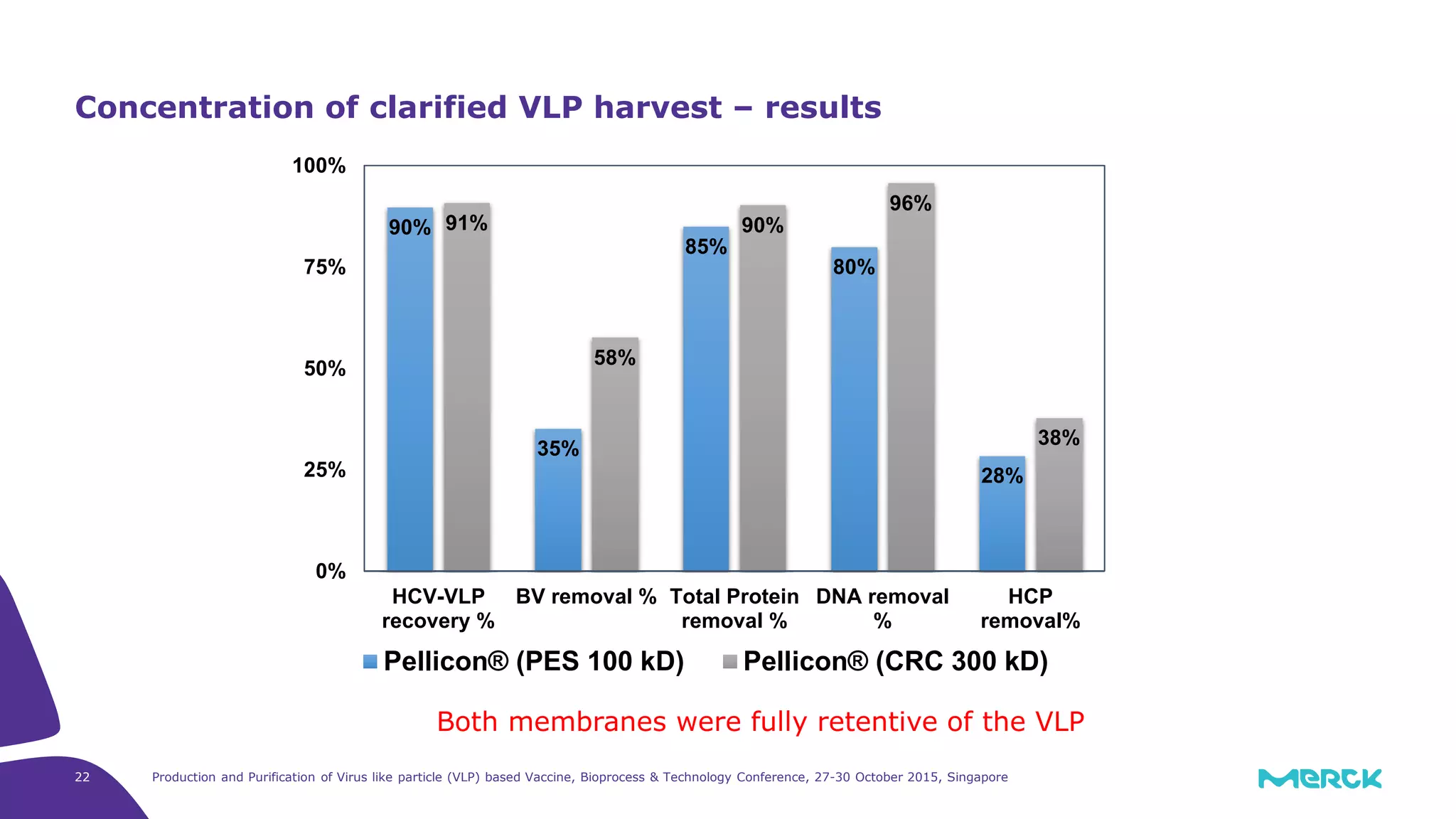
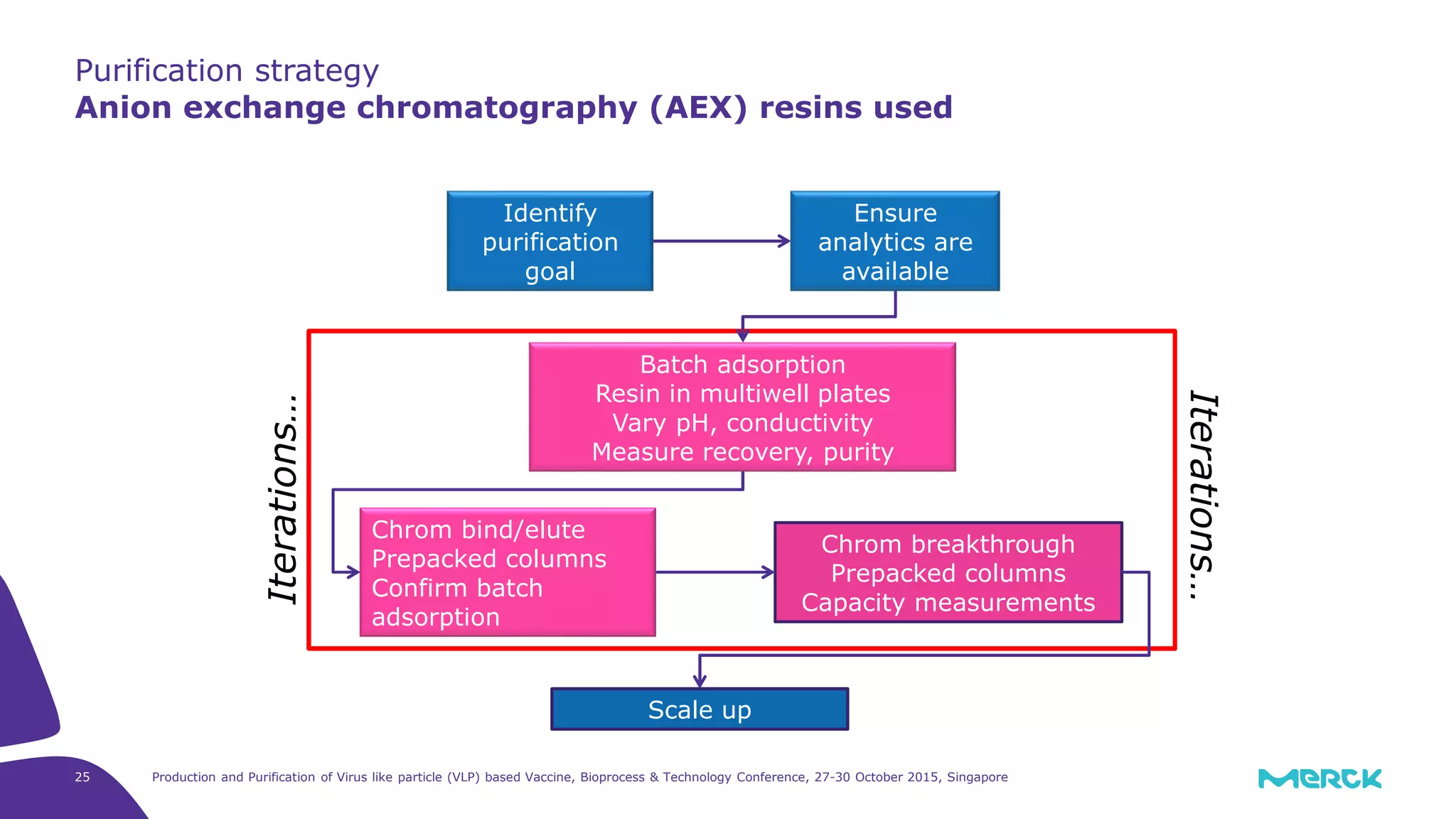
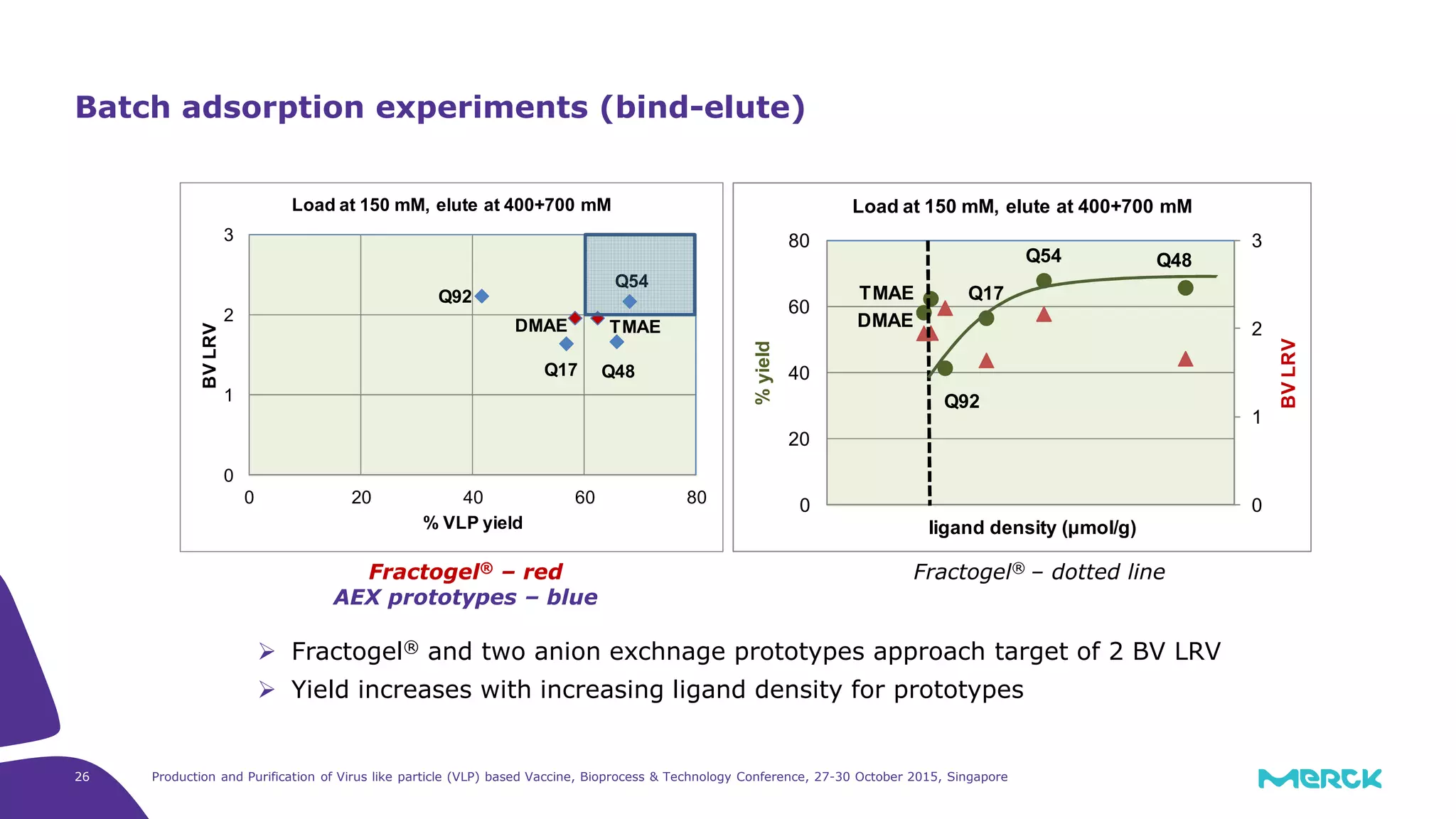
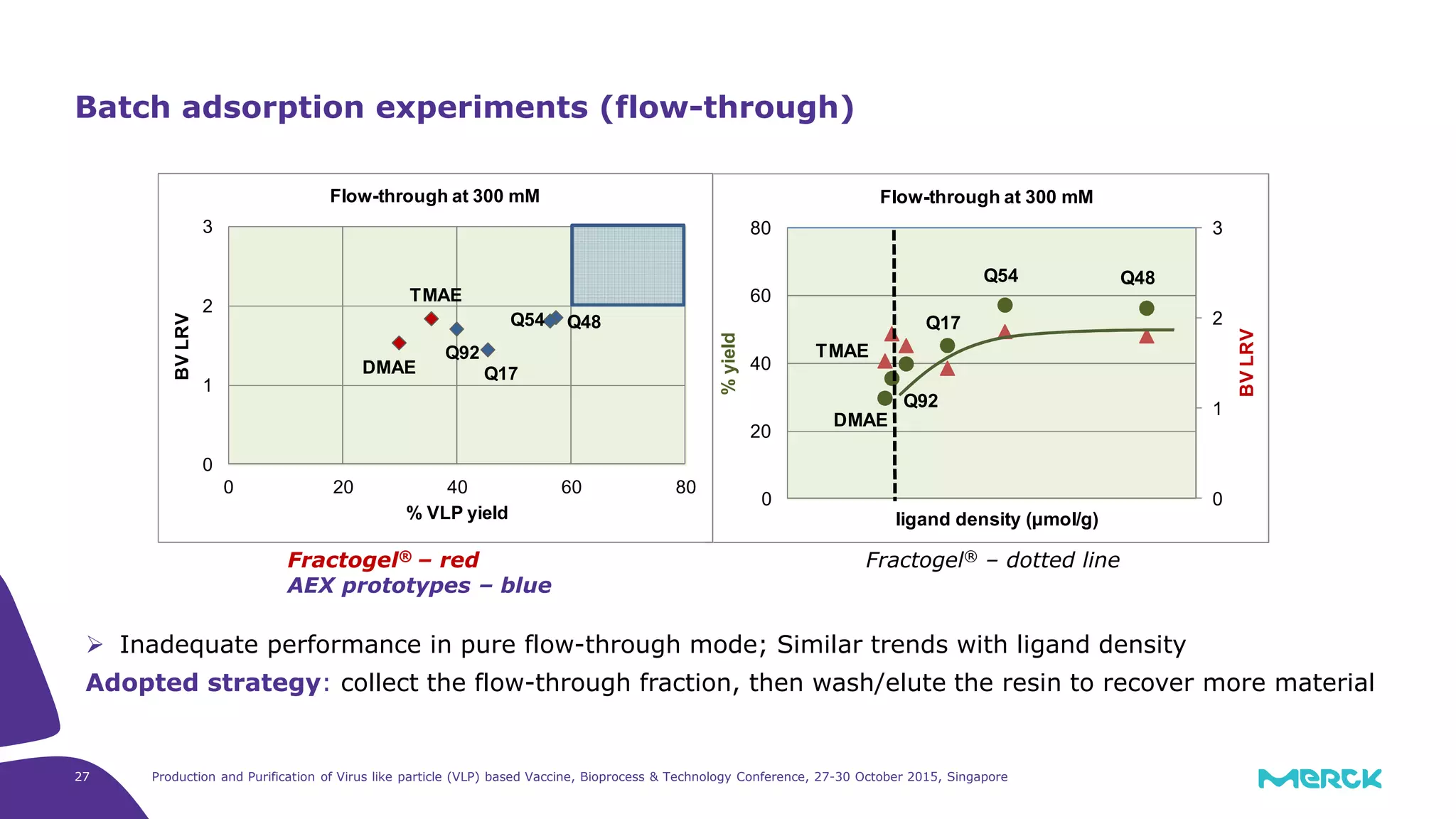
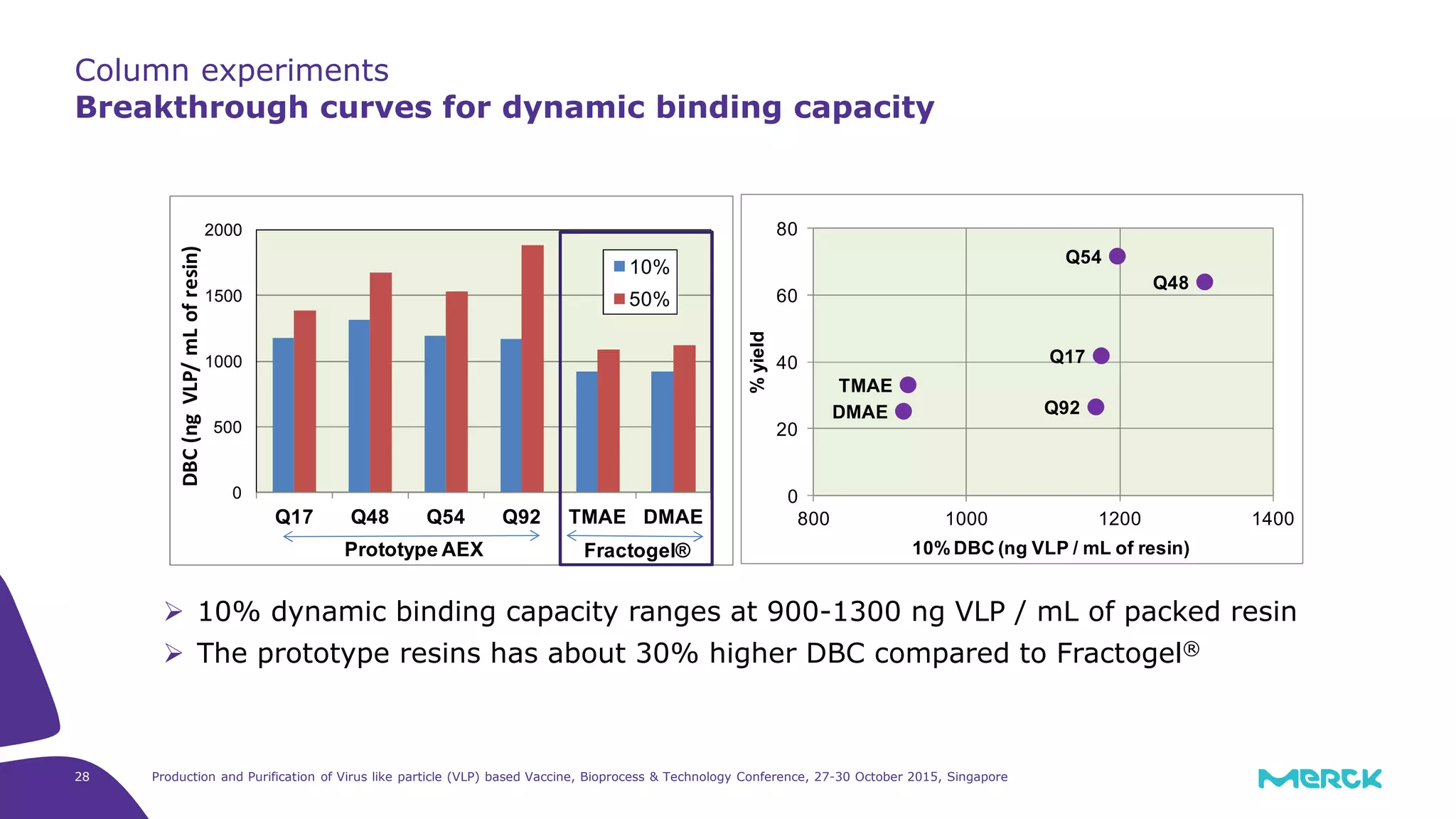
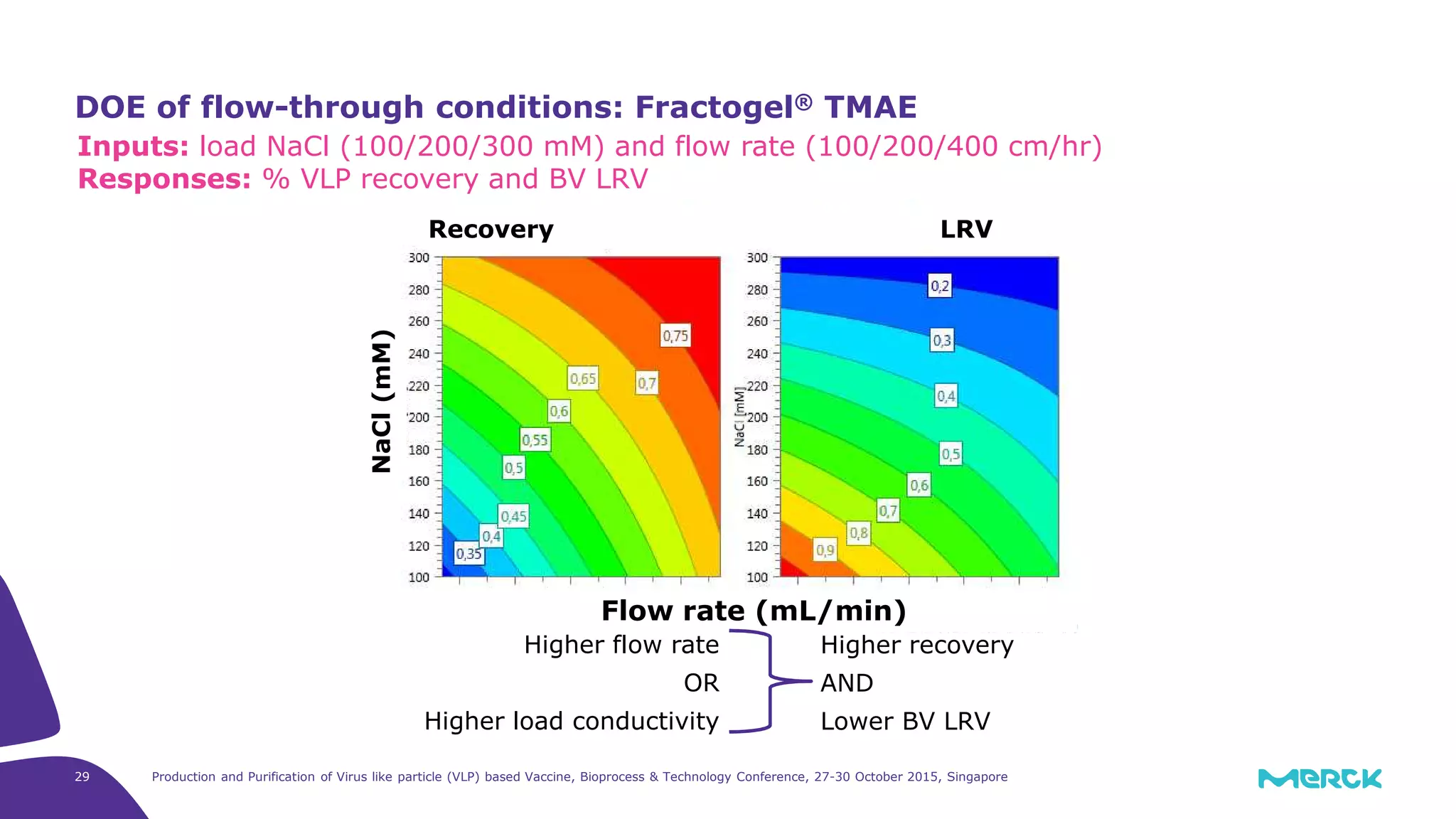
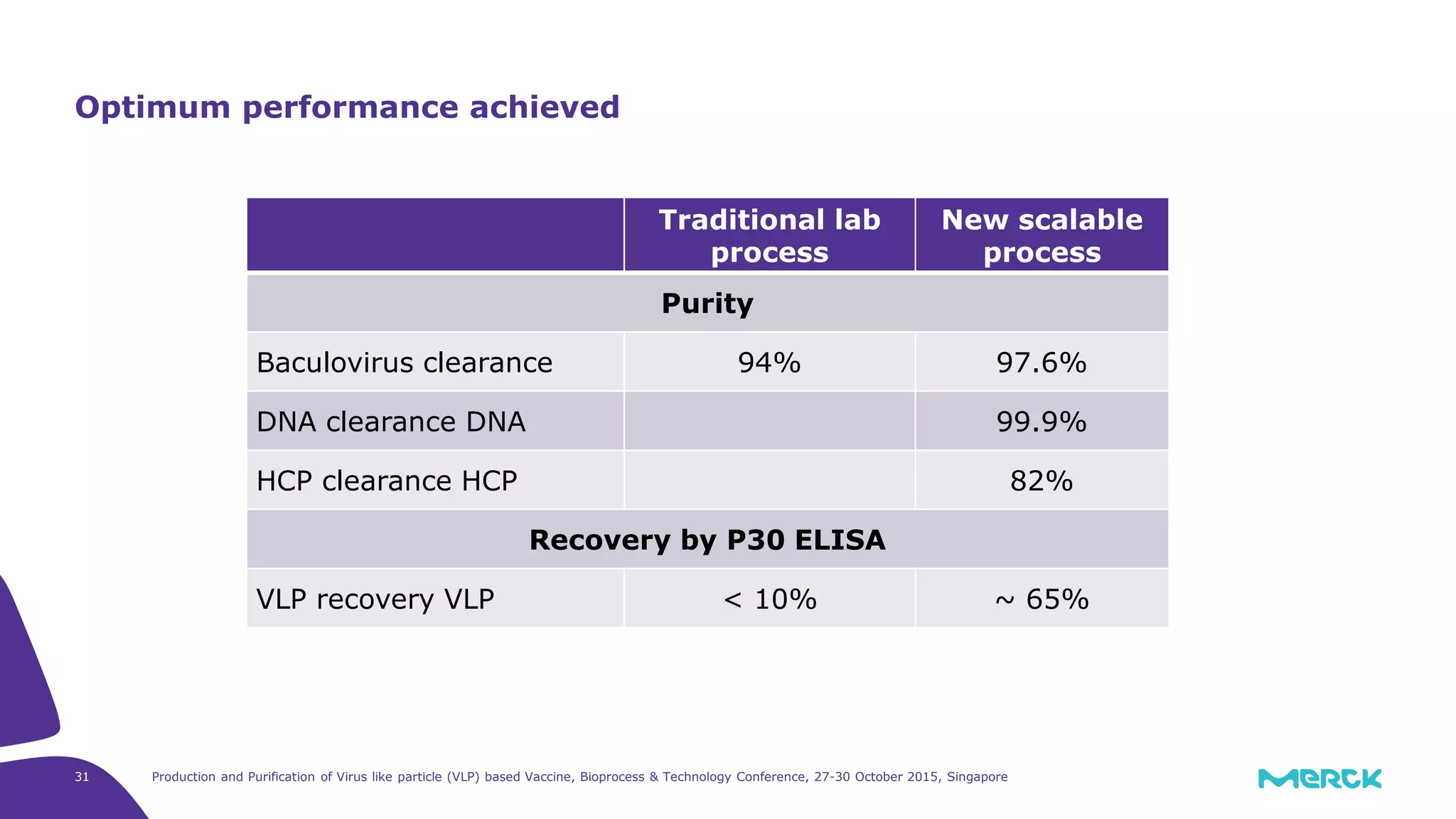
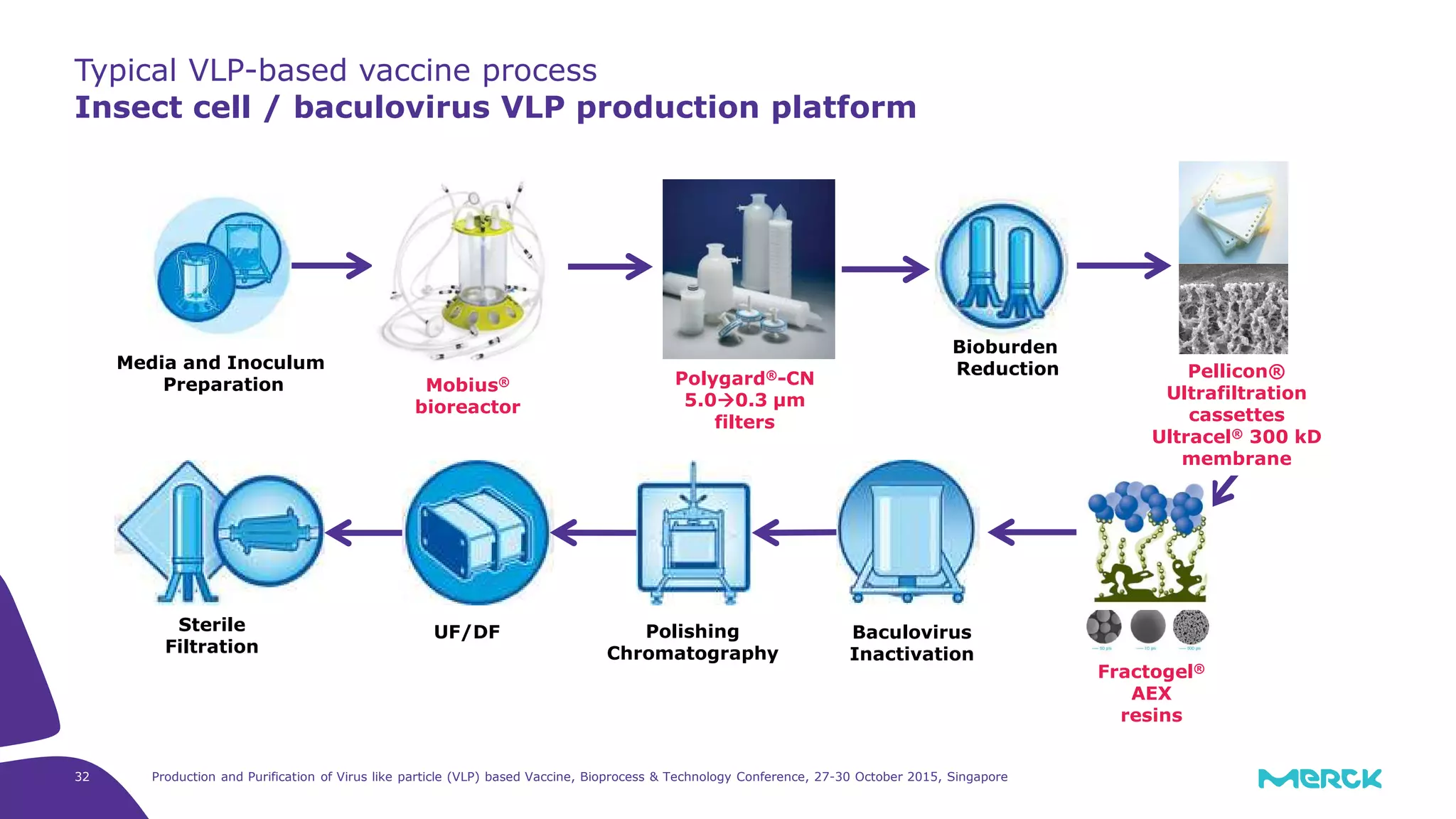
This document summarizes a presentation on the production and purification of virus-like particle (VLP) based vaccines. It discusses using hepatitis C VLPs as a model system produced using an insect cell/baculovirus expression platform. Key points covered include:
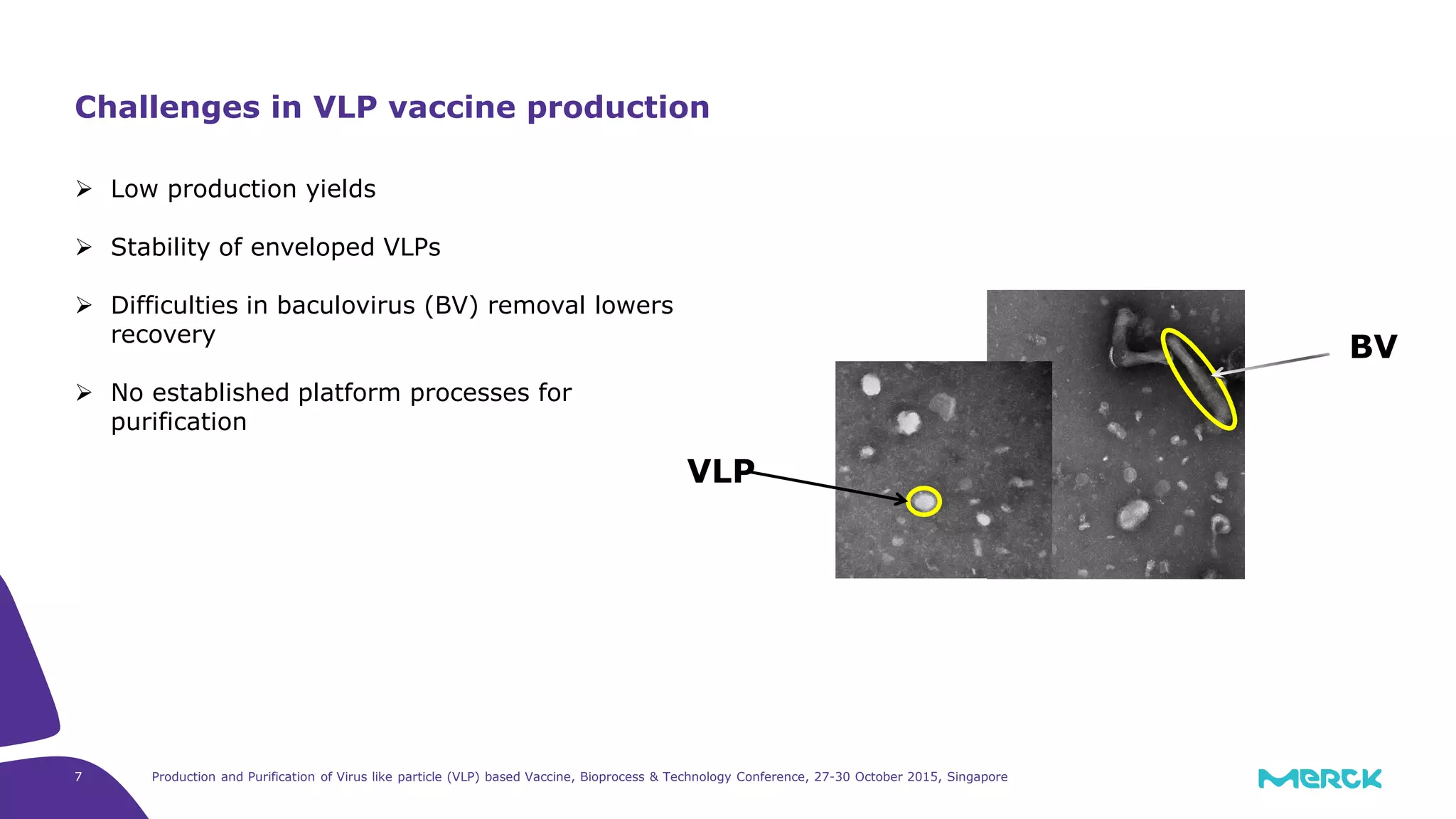
- Challenges in VLP vaccine production include low yields, stability issues, and difficulties removing baculovirus.
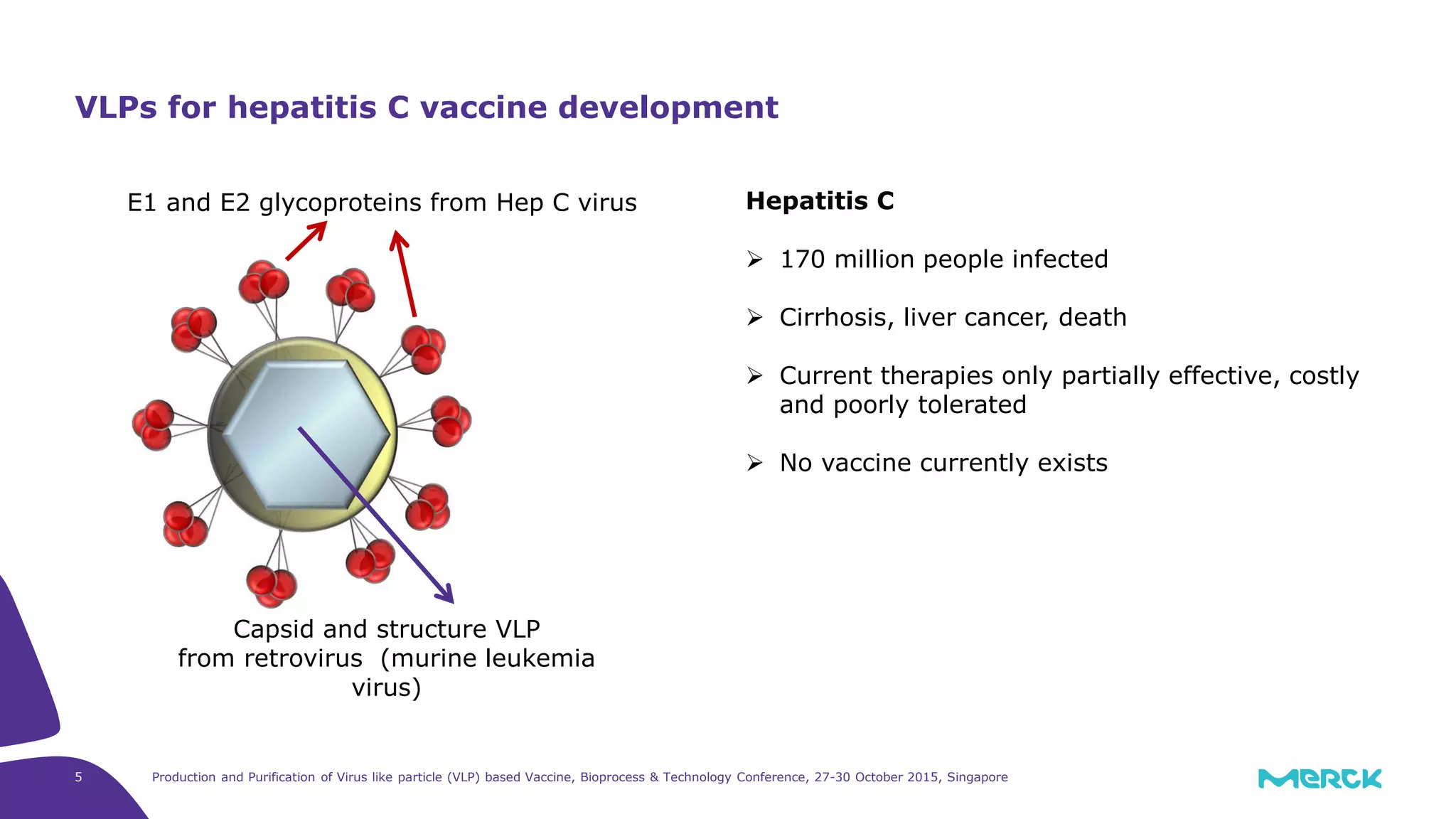
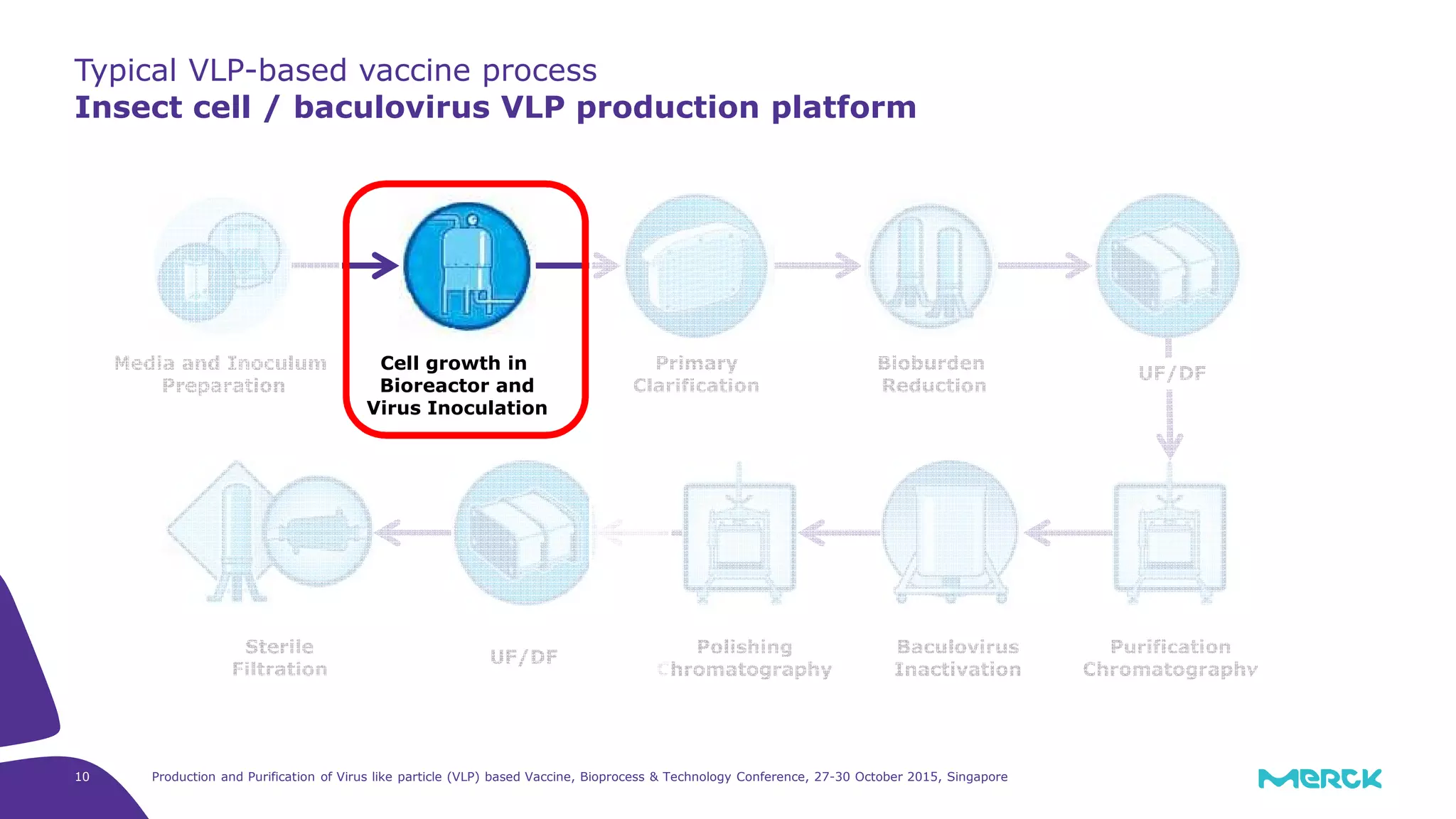
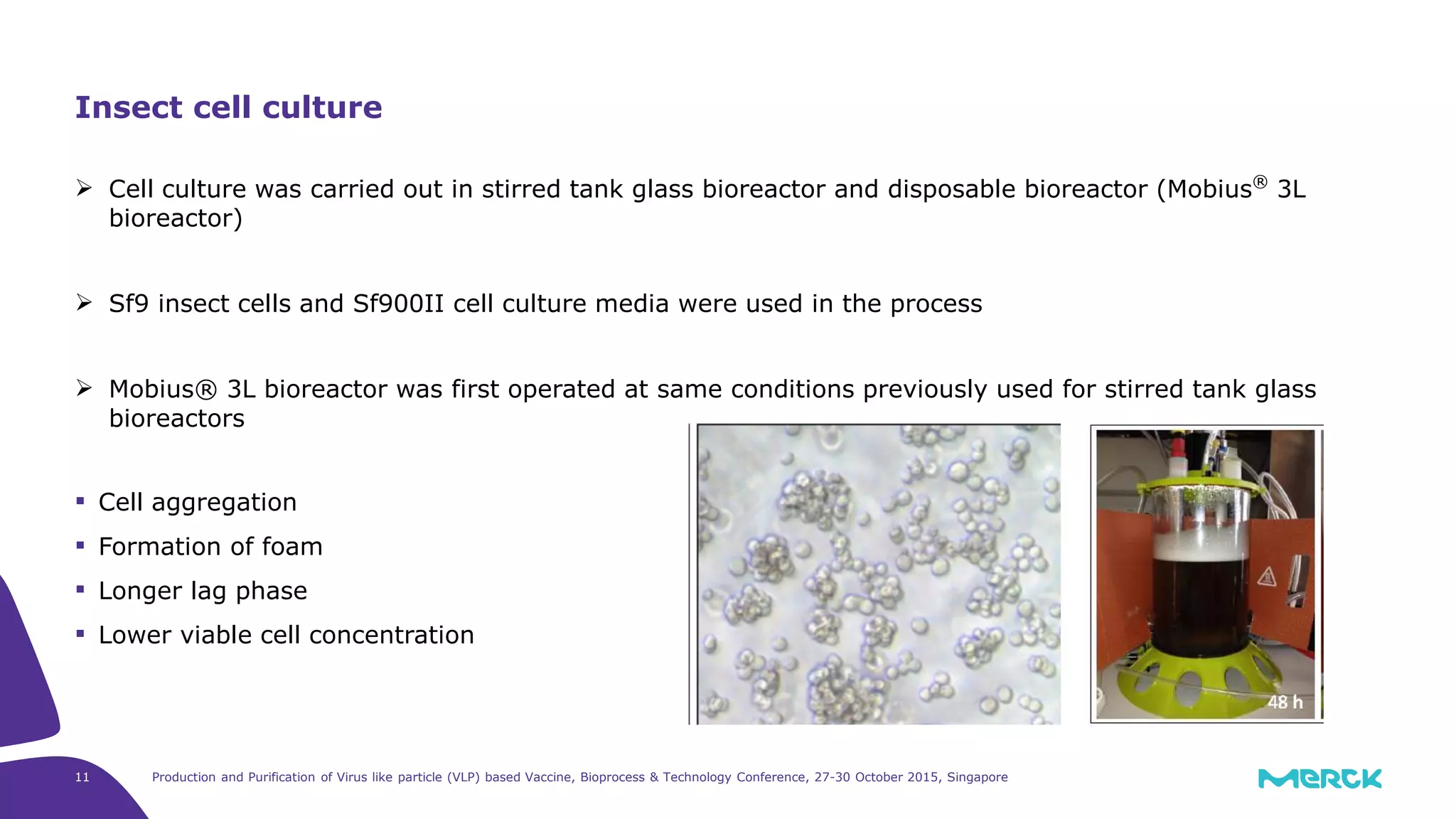
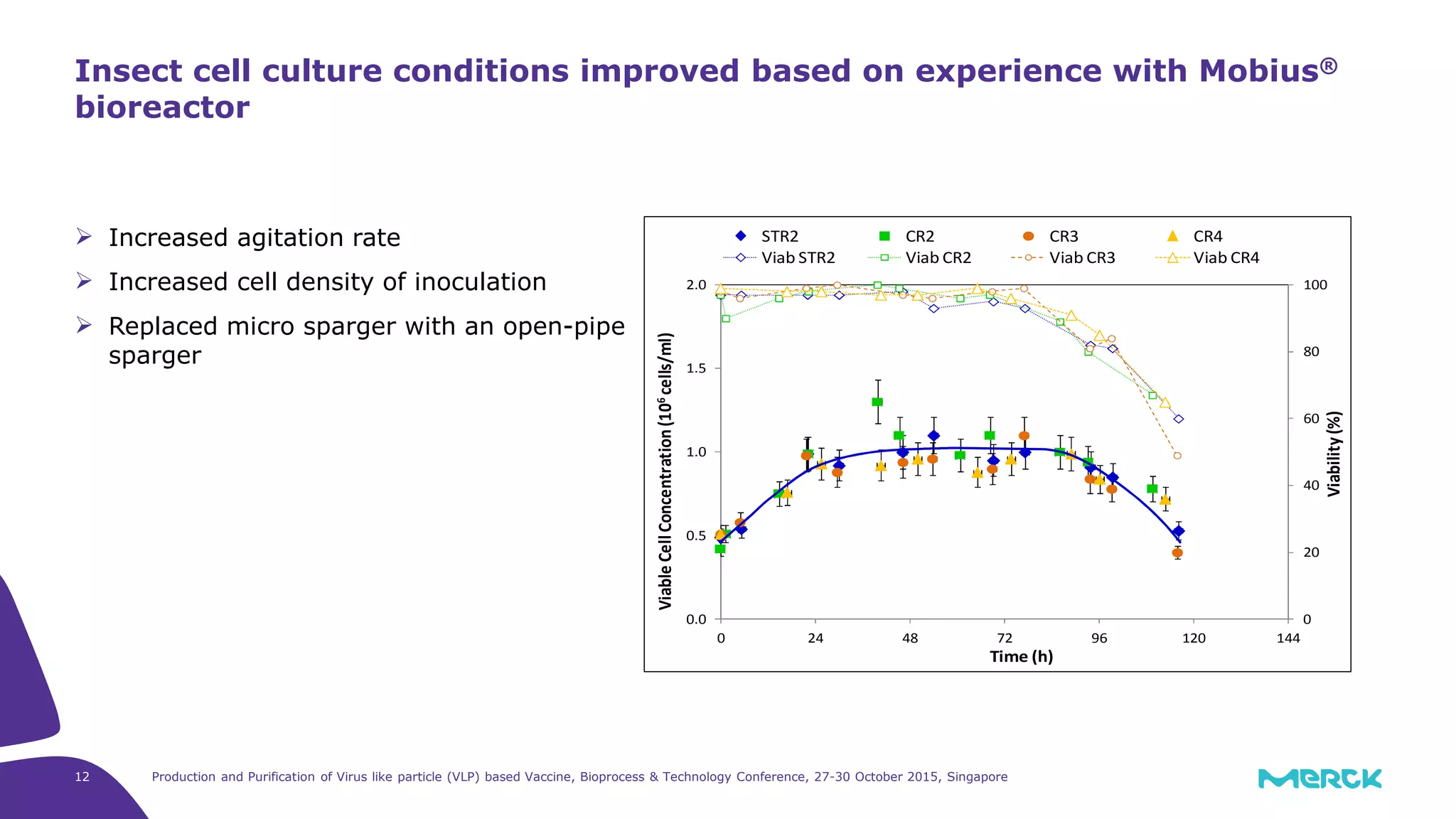
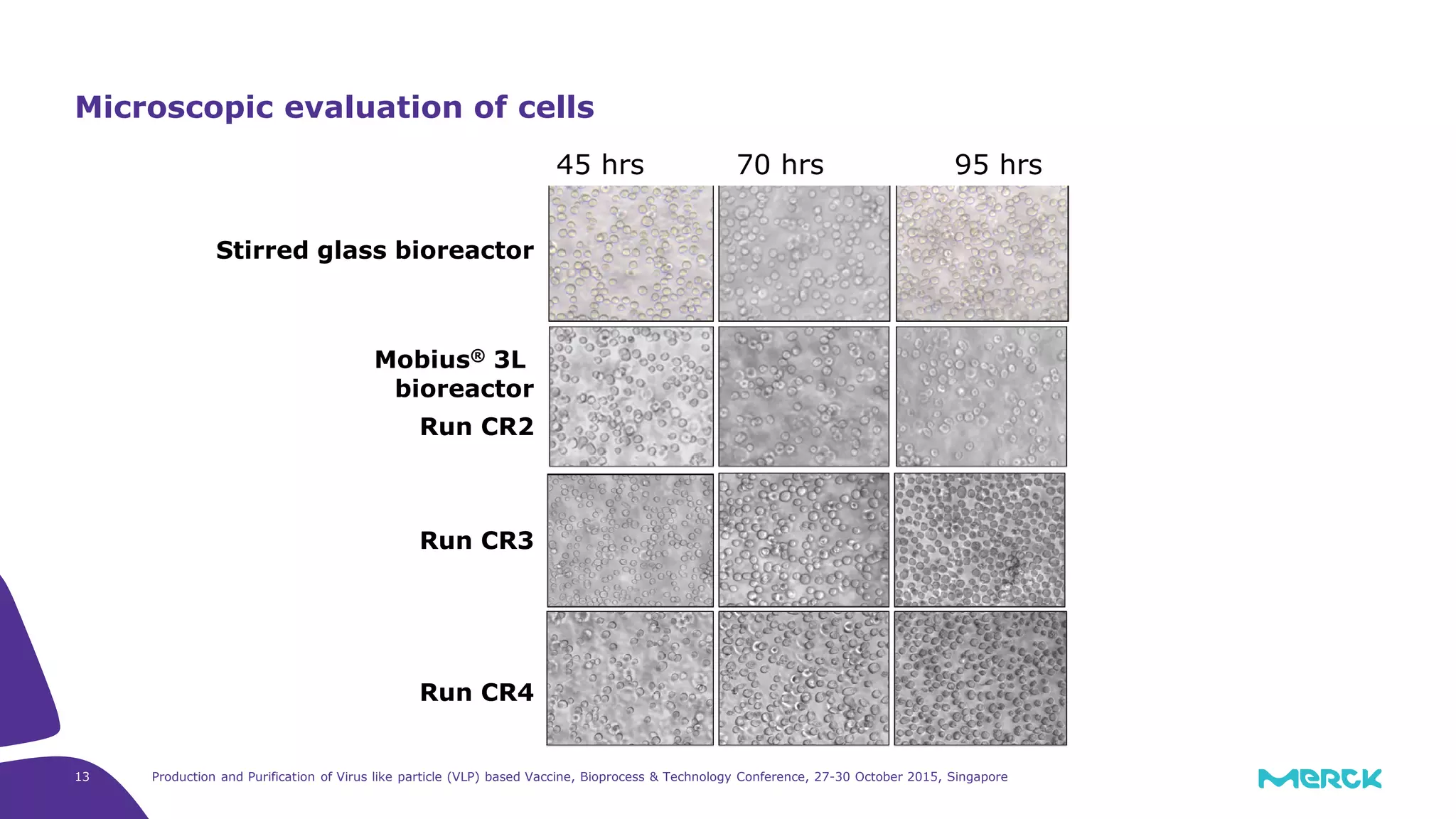
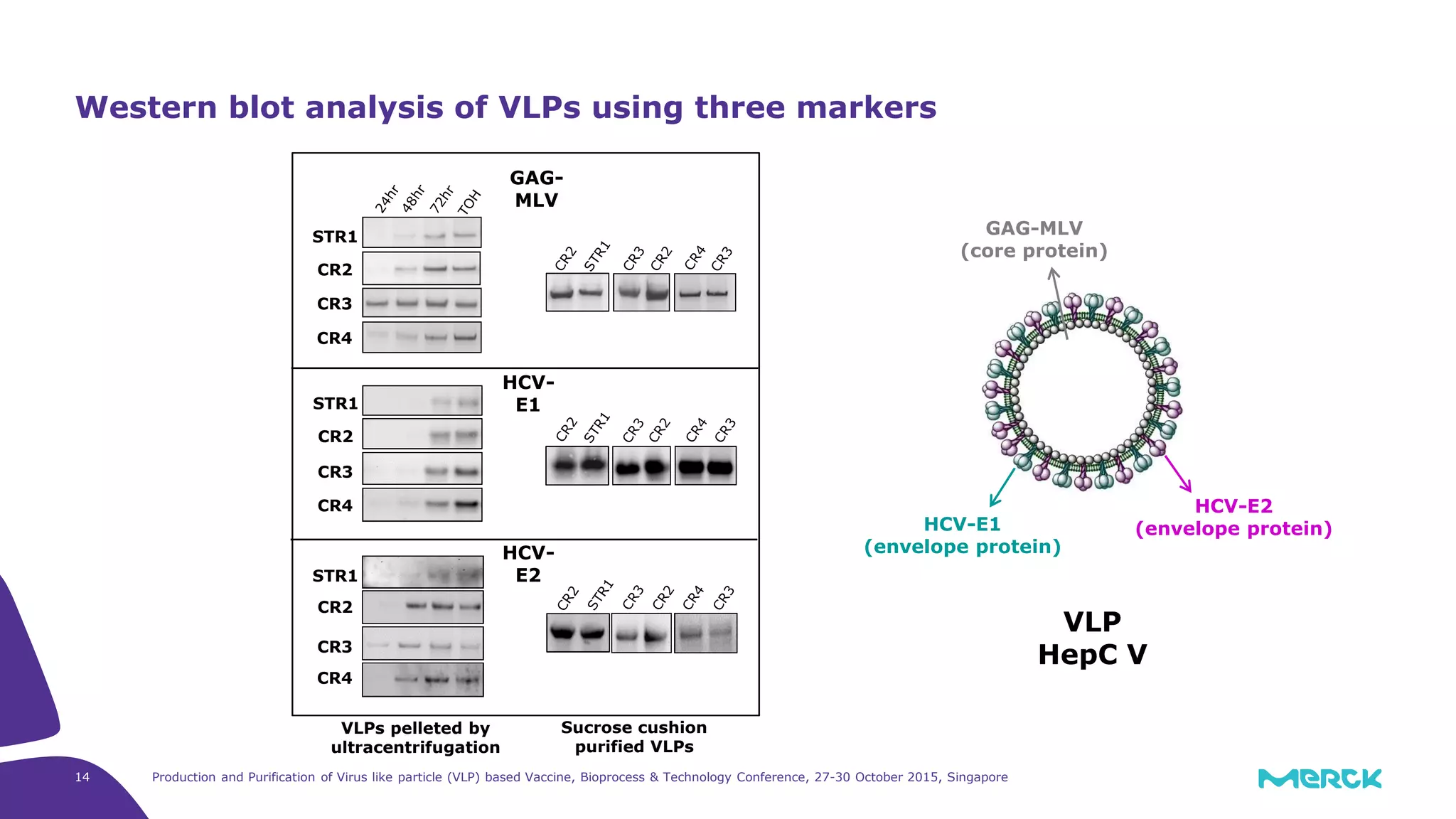
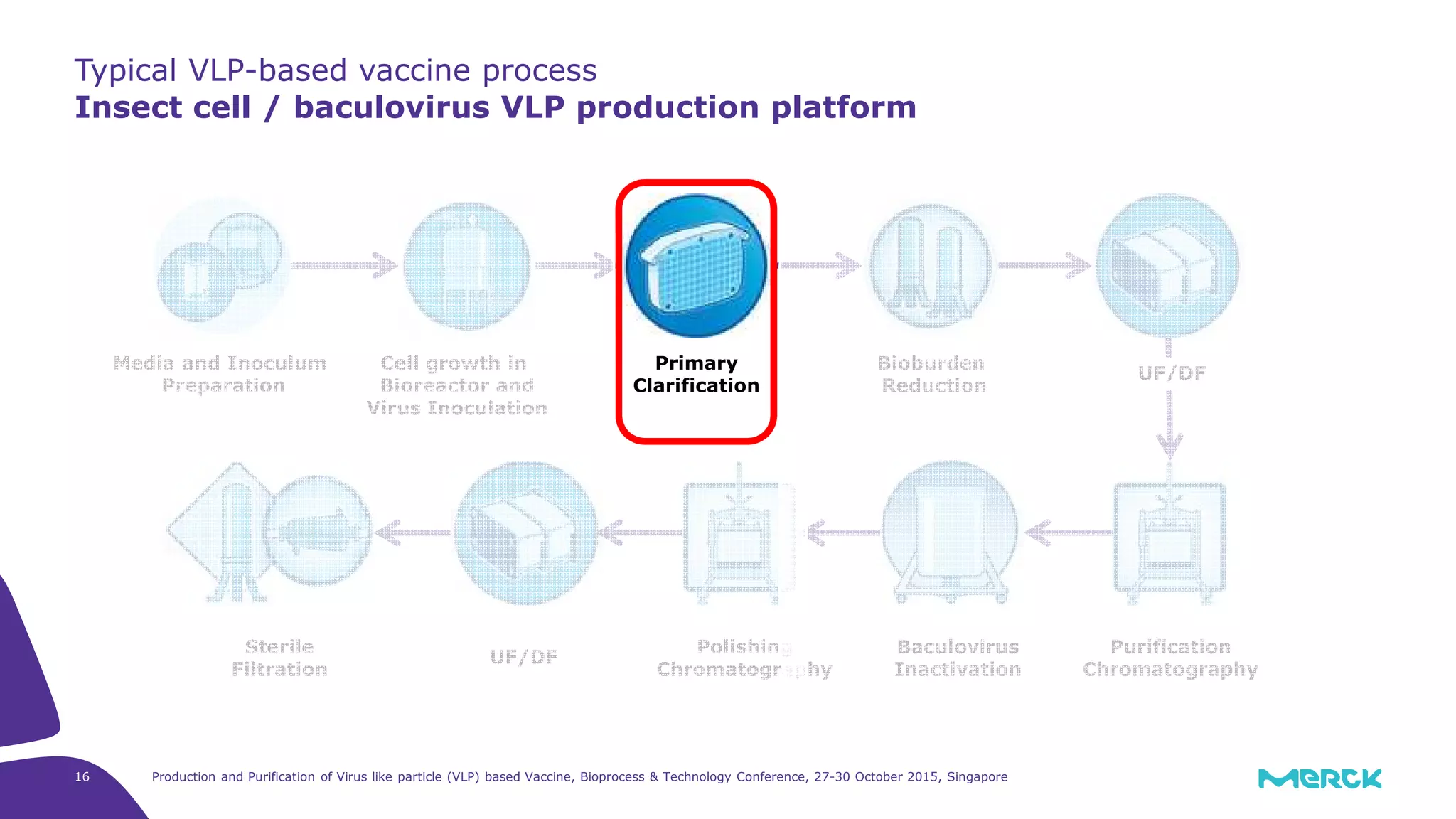
- Hepatitis C VLPs containing E1 and E2 glycoproteins were successfully produced using Sf9 insect cells in a Mobius 3L disposable bioreactor, with comparable results to a glass bioreactor.
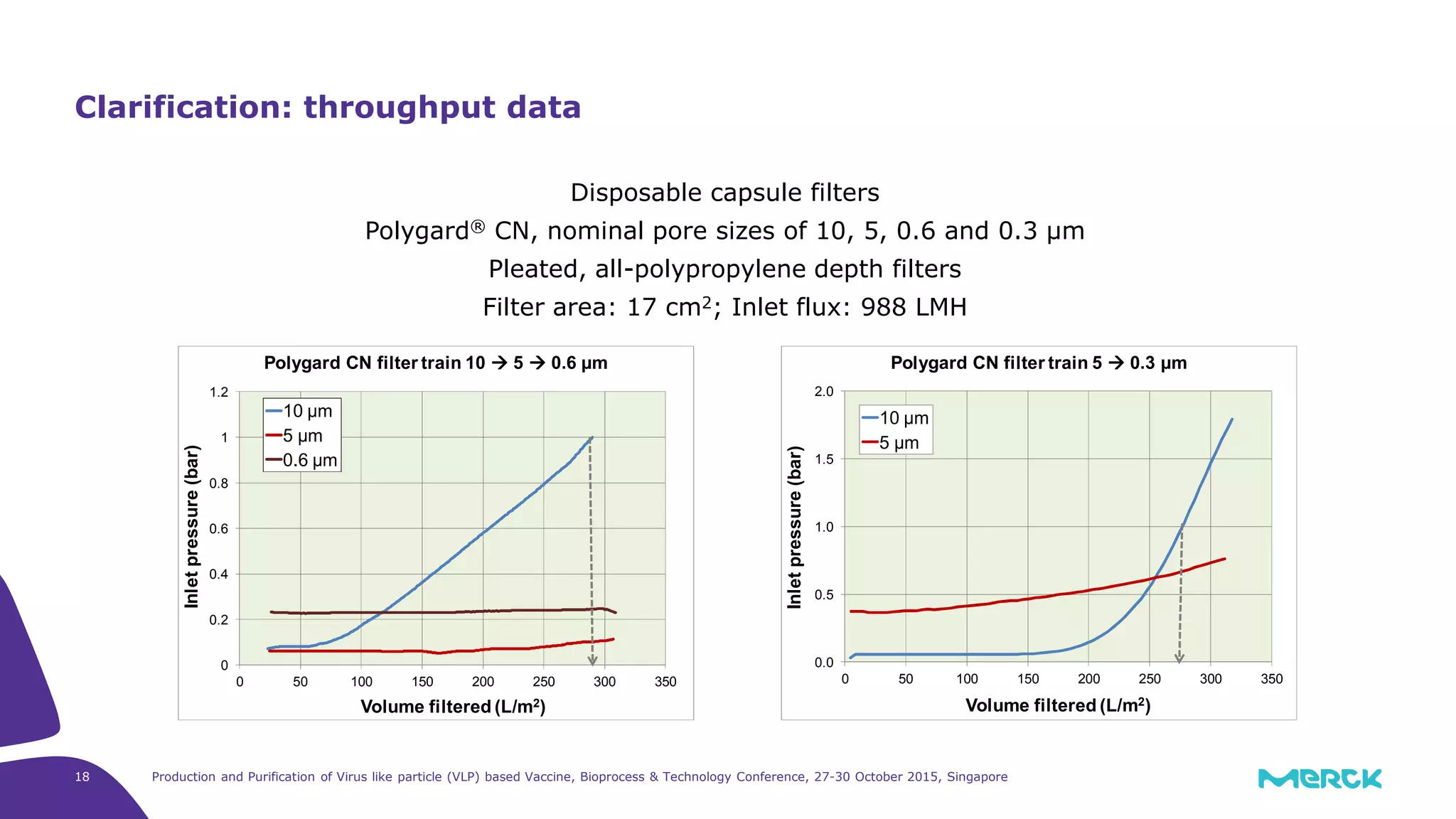
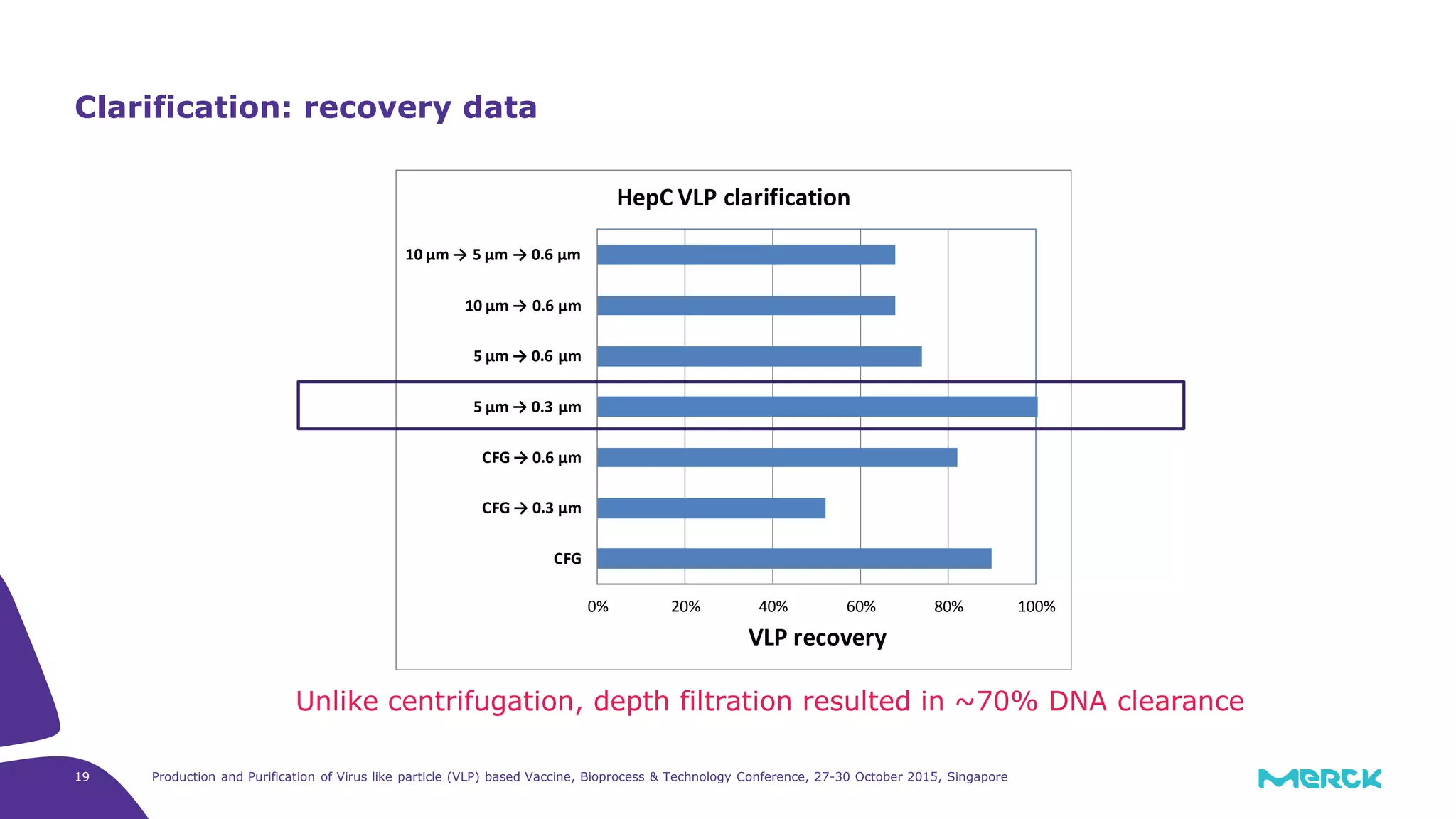
- A depth filtration clarification process achieved around 70% DNA clearance while recovering approximately 70