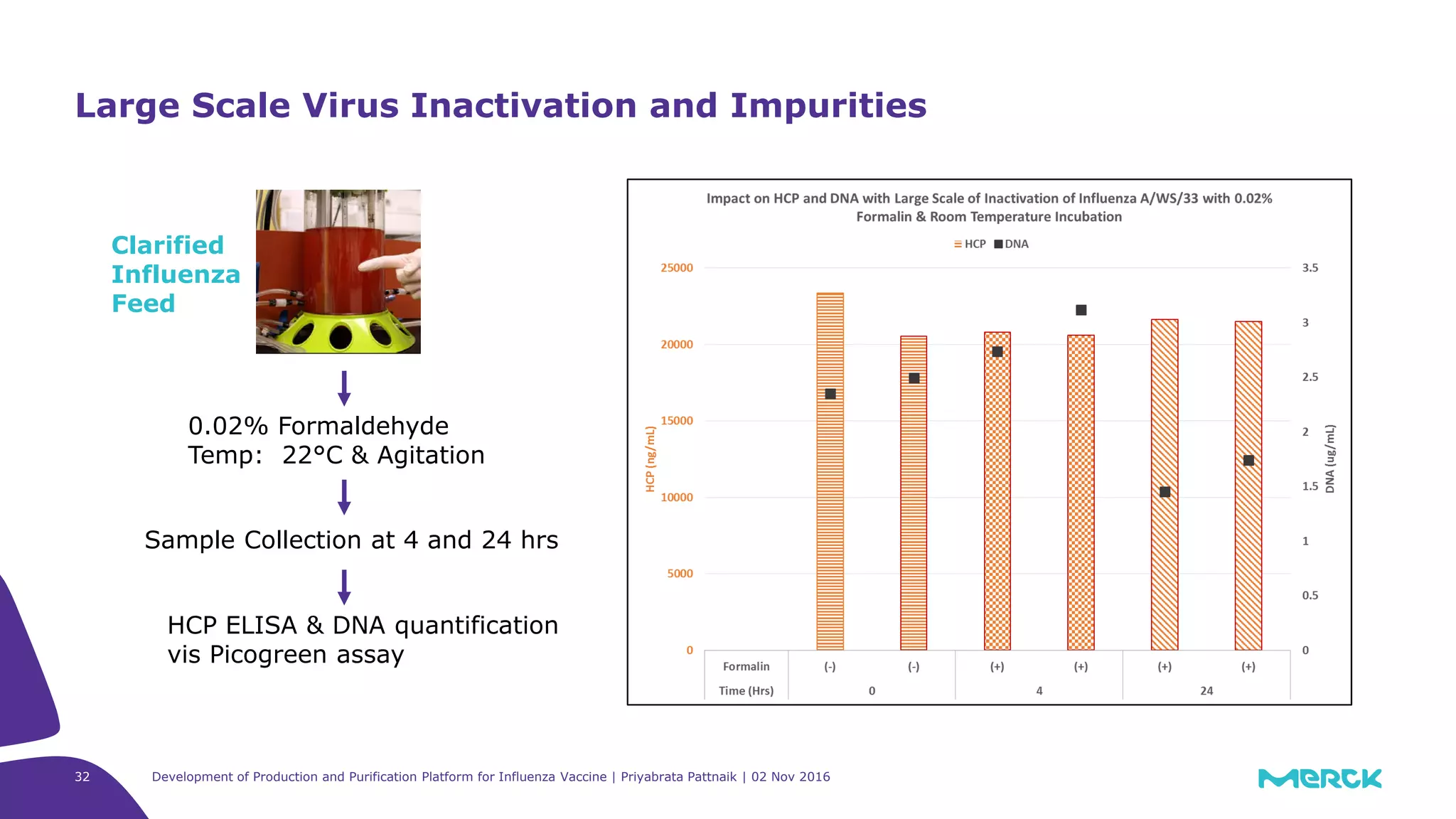
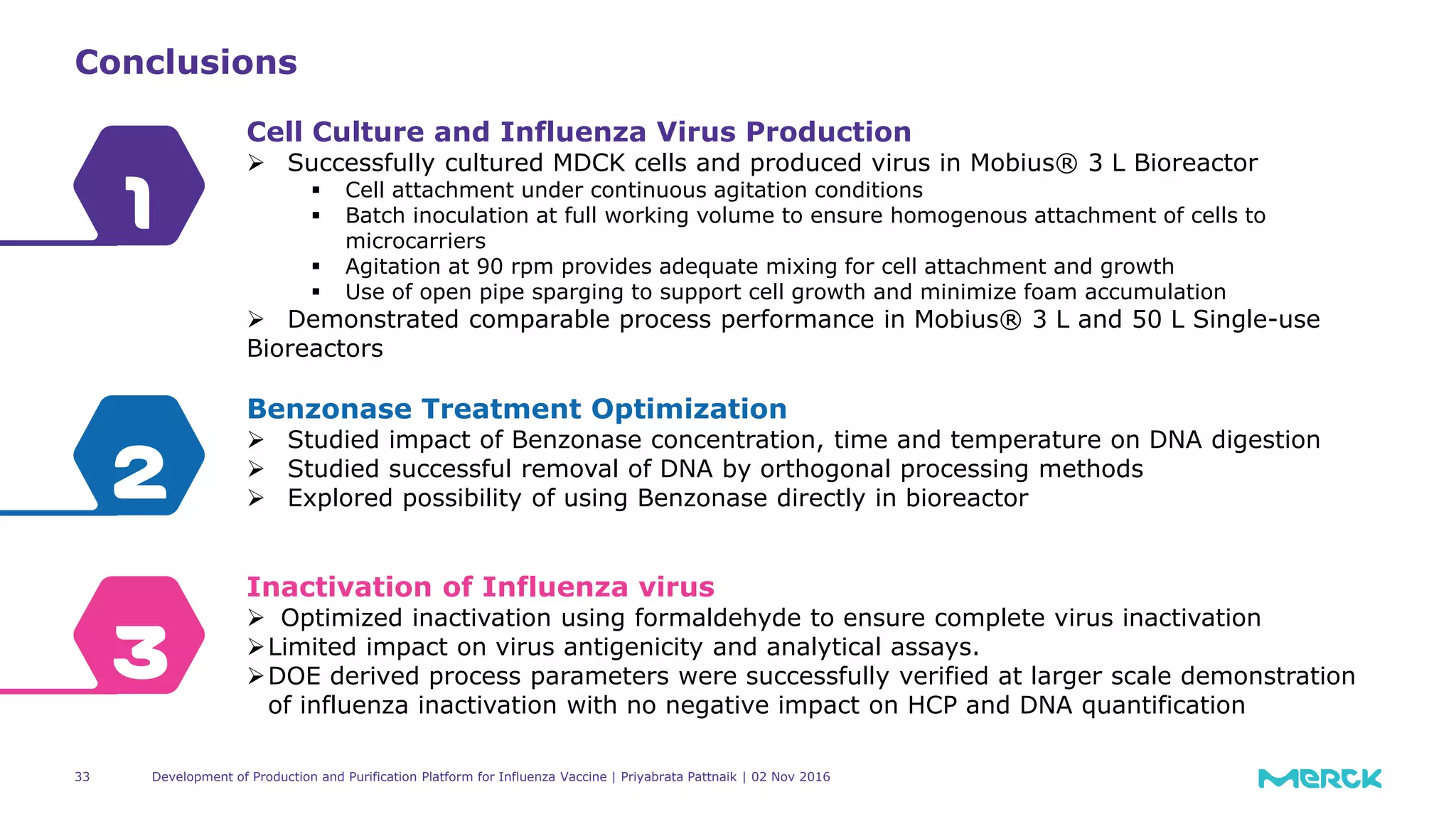
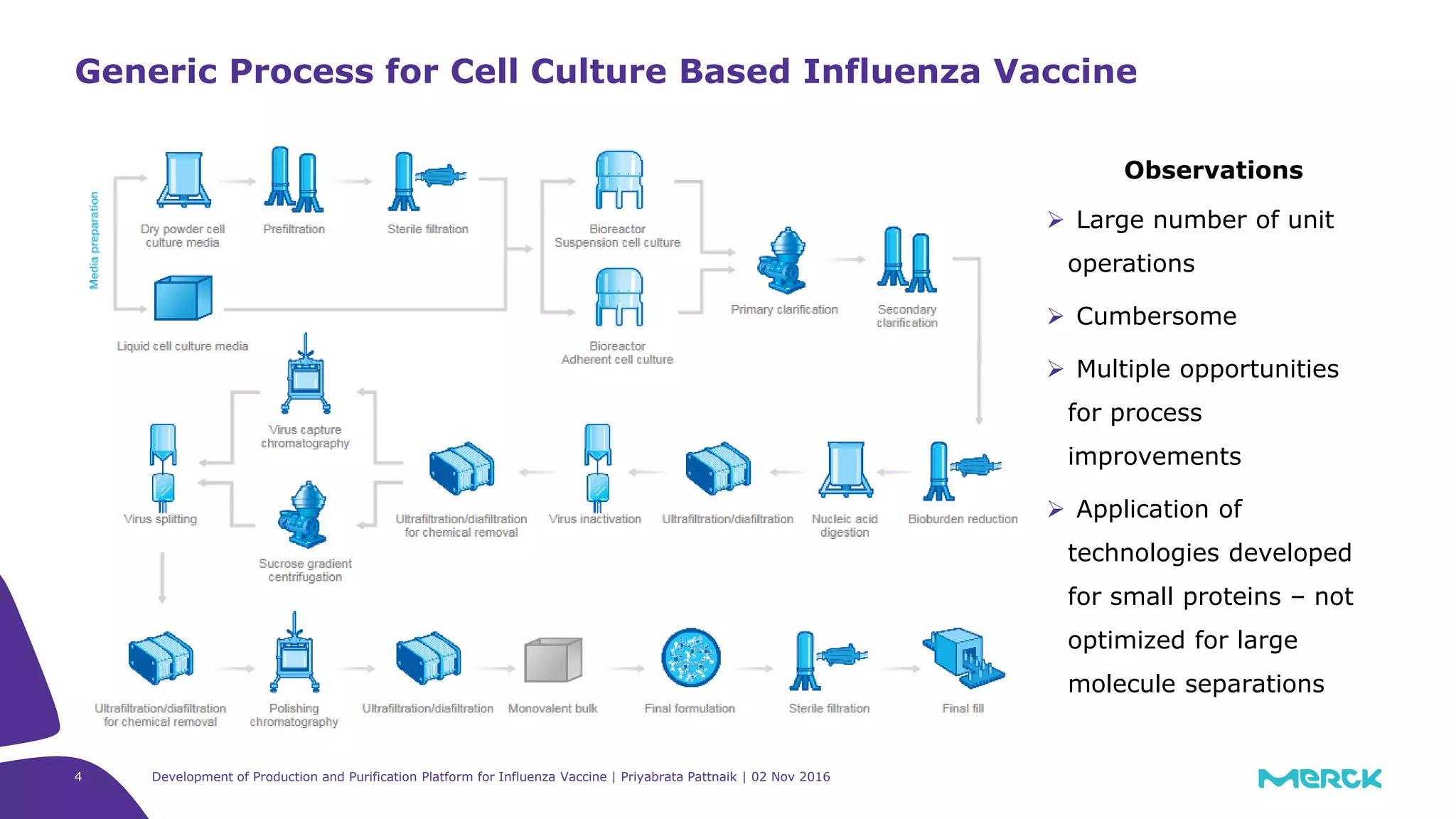
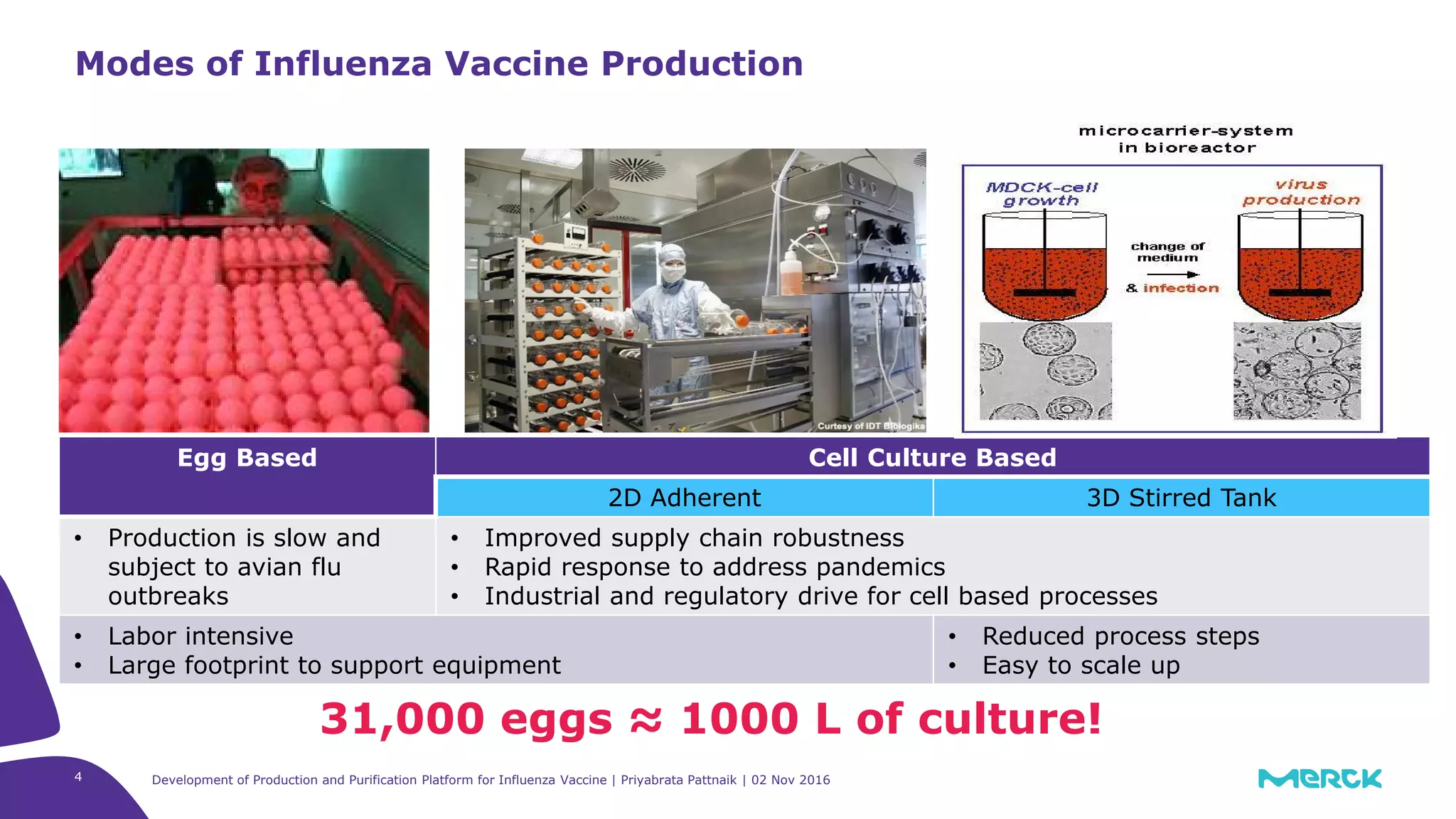
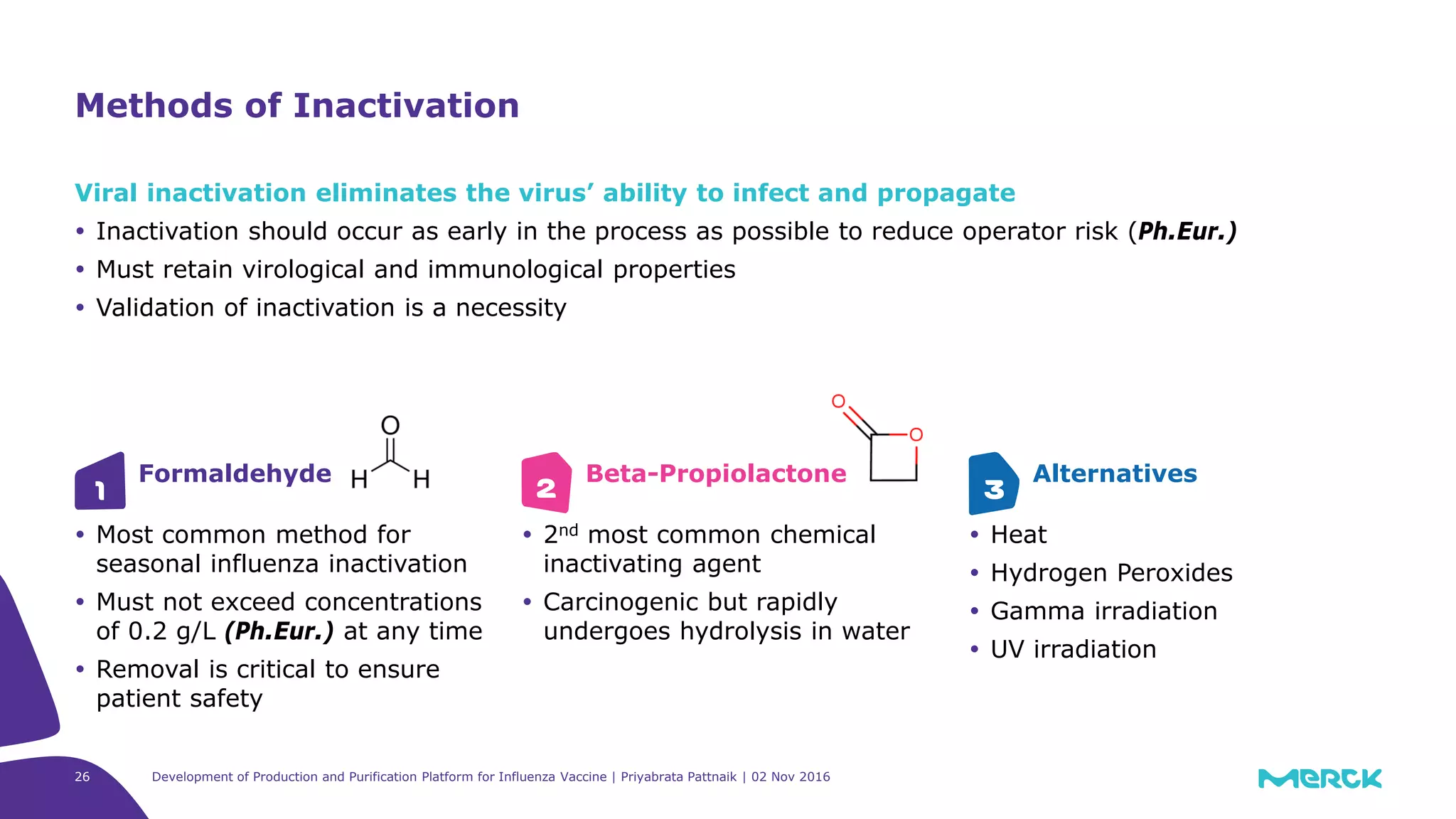
The document details the development of a production and purification platform for influenza vaccines, including upstream virus production, DNA removal using benzonase® treatment, and formaldehyde-based virus inactivation methods. It highlights improvements in cell culture techniques, bioreactor optimization, and the effects of various parameters on productivity and safety. Conclusions emphasize the effectiveness of process enhancements and the significance of monitoring viral inactivation to ensure patient safety.


![Annual Vaccine
HA protein (most common)
Typically 3-4 varieties (valences)
Dosage info (0.5mL/dose)
− HA: 15μg of each HA protein
− Formaldehyde: ≤ 200ppm
− Endotoxin: < 100 IU/dose
− Other protein: < 6 x HA content (< 300 μg/dose)
− DNA: < 10ng/dose
− Purity: 95% by gel (Coomassie blue)
Background
Influenza
3
80-120 nm enveloped virus
By National Institutes of Health; originally uploaded to en.wikipedia by TimVickers (25 October 2006),
transferred to Commons by Quadell using CommonsHelper. (California Department of Health Services) [Public
domain], via Wikimedia Commons
Development of Production and Purification Platform for Influenza Vaccine | Priyabrata Pattnaik | 02 Nov 2016](https://image.slidesharecdn.com/devofprodpurificplatformforinfluenzavaccinepattnaik-161115141406/75/Development-of-Production-and-Purification-Platformform-for-Influenza-Vaccine-3-2048.jpg)

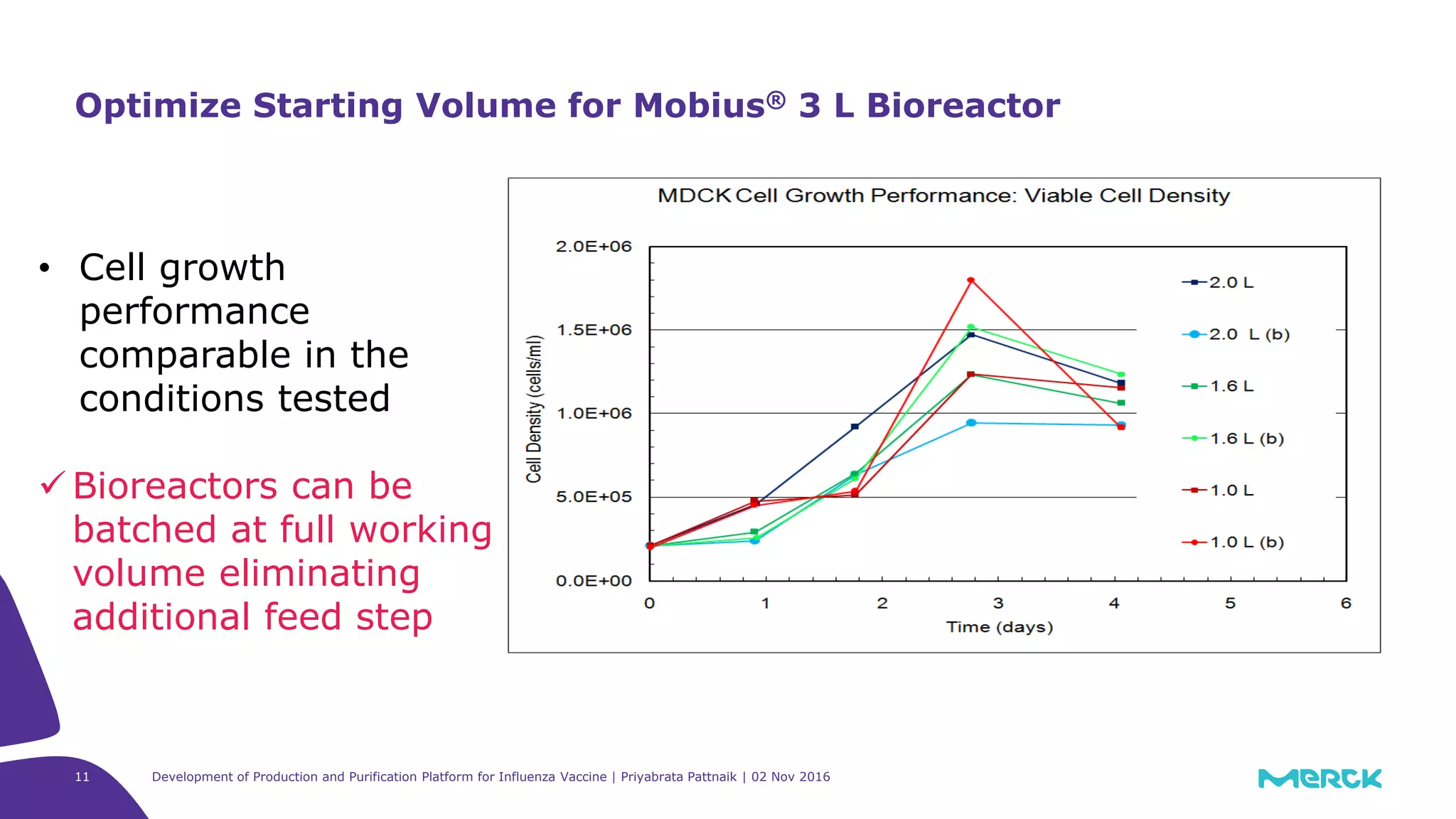
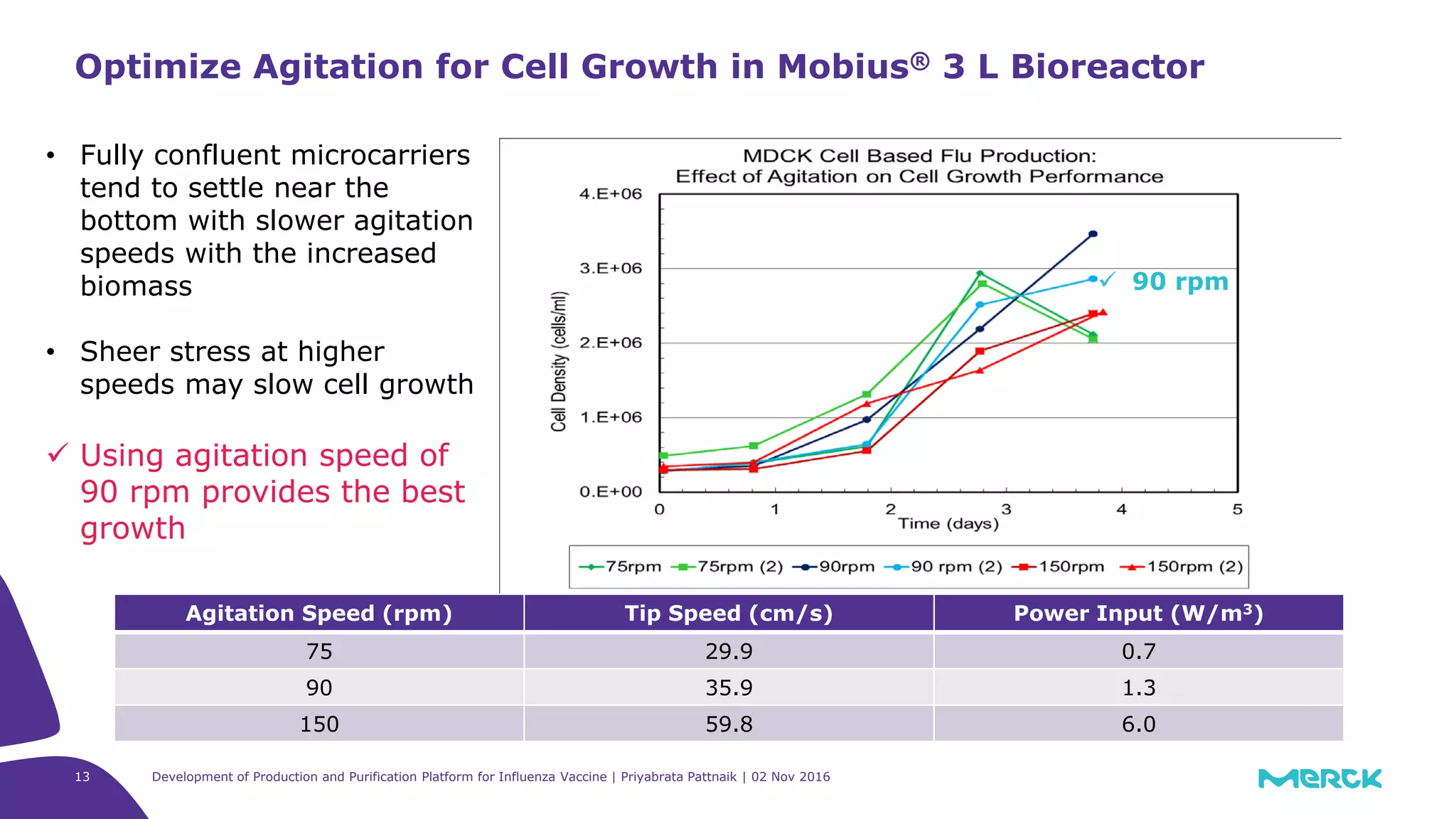

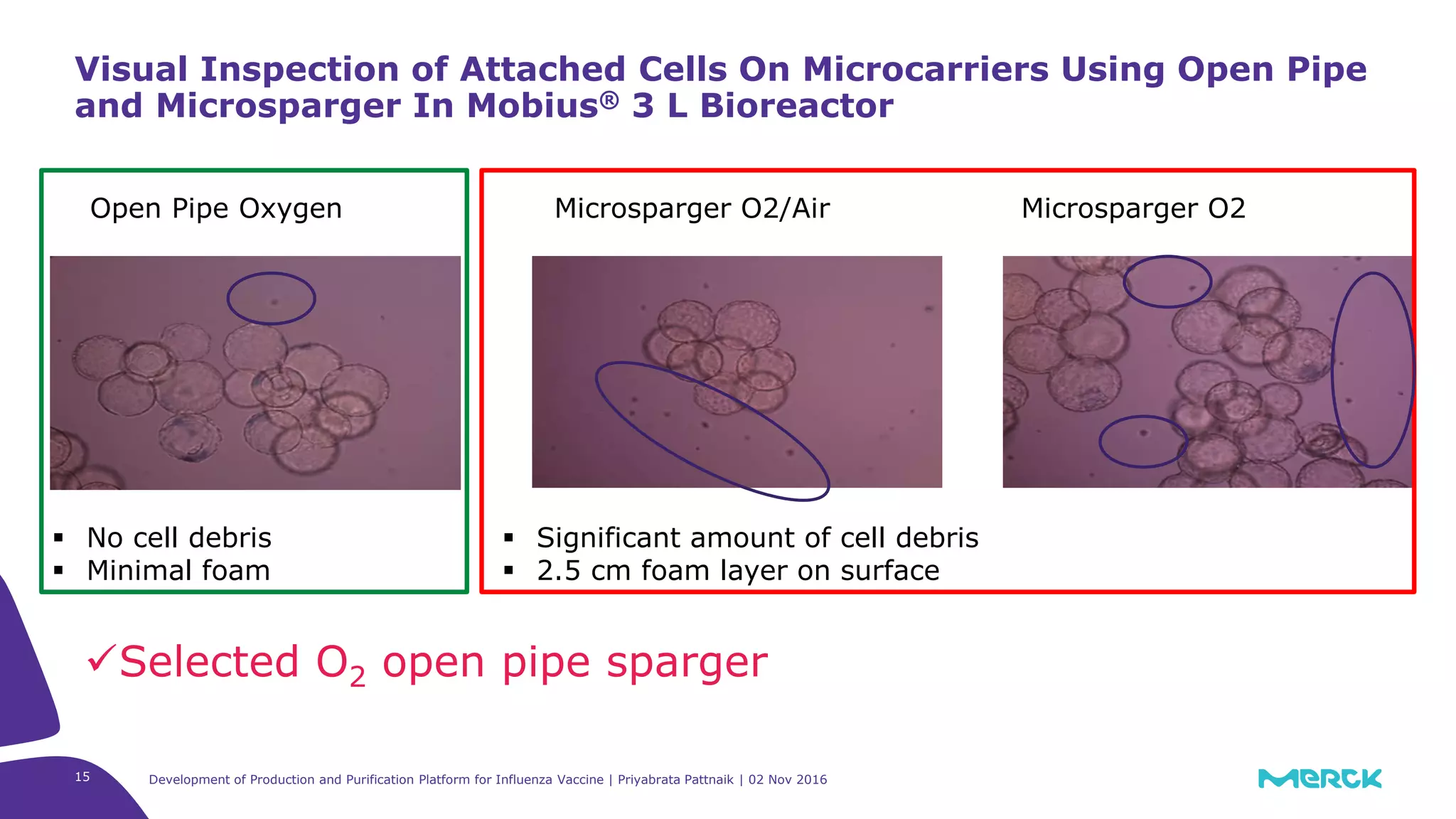
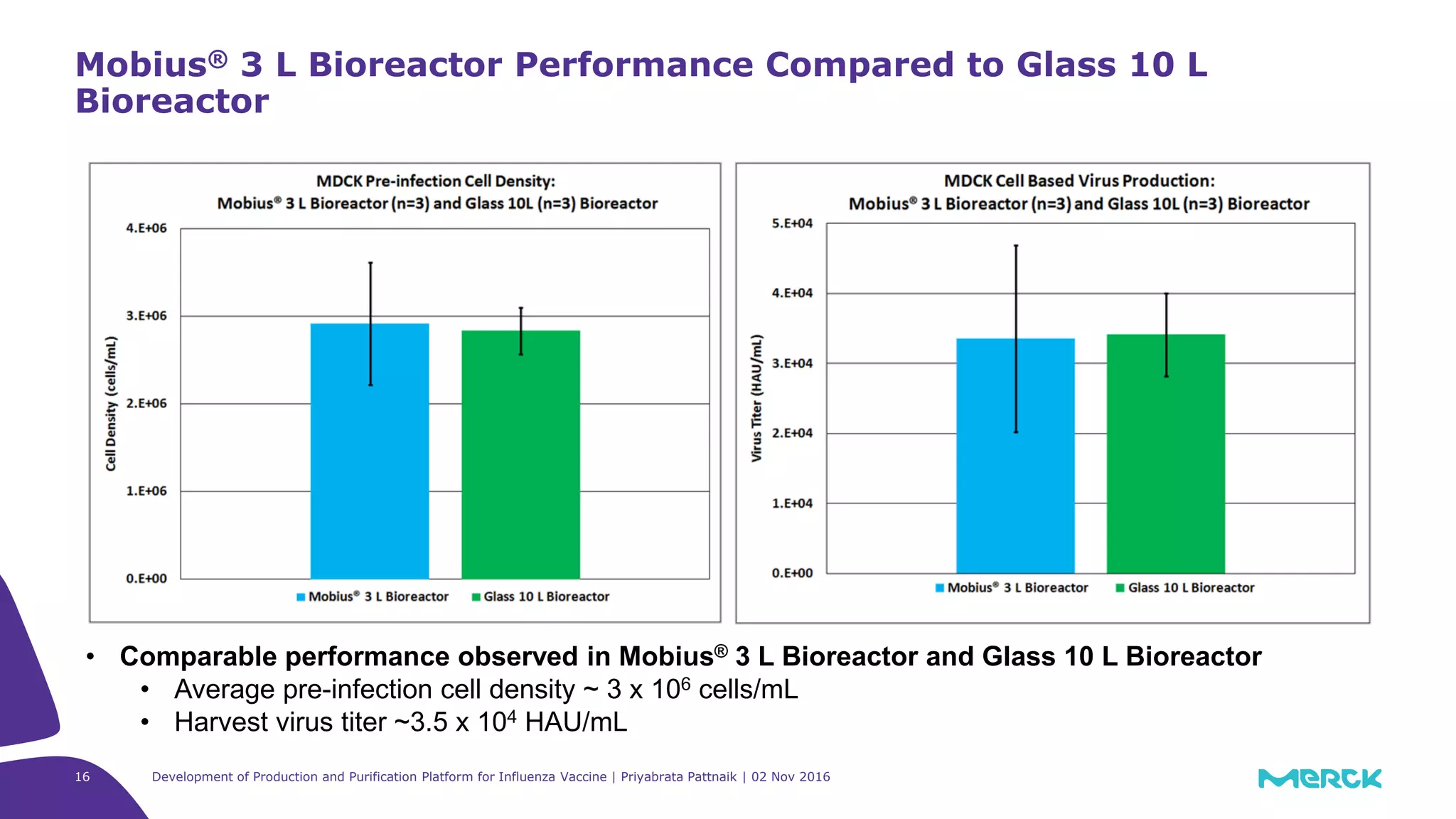
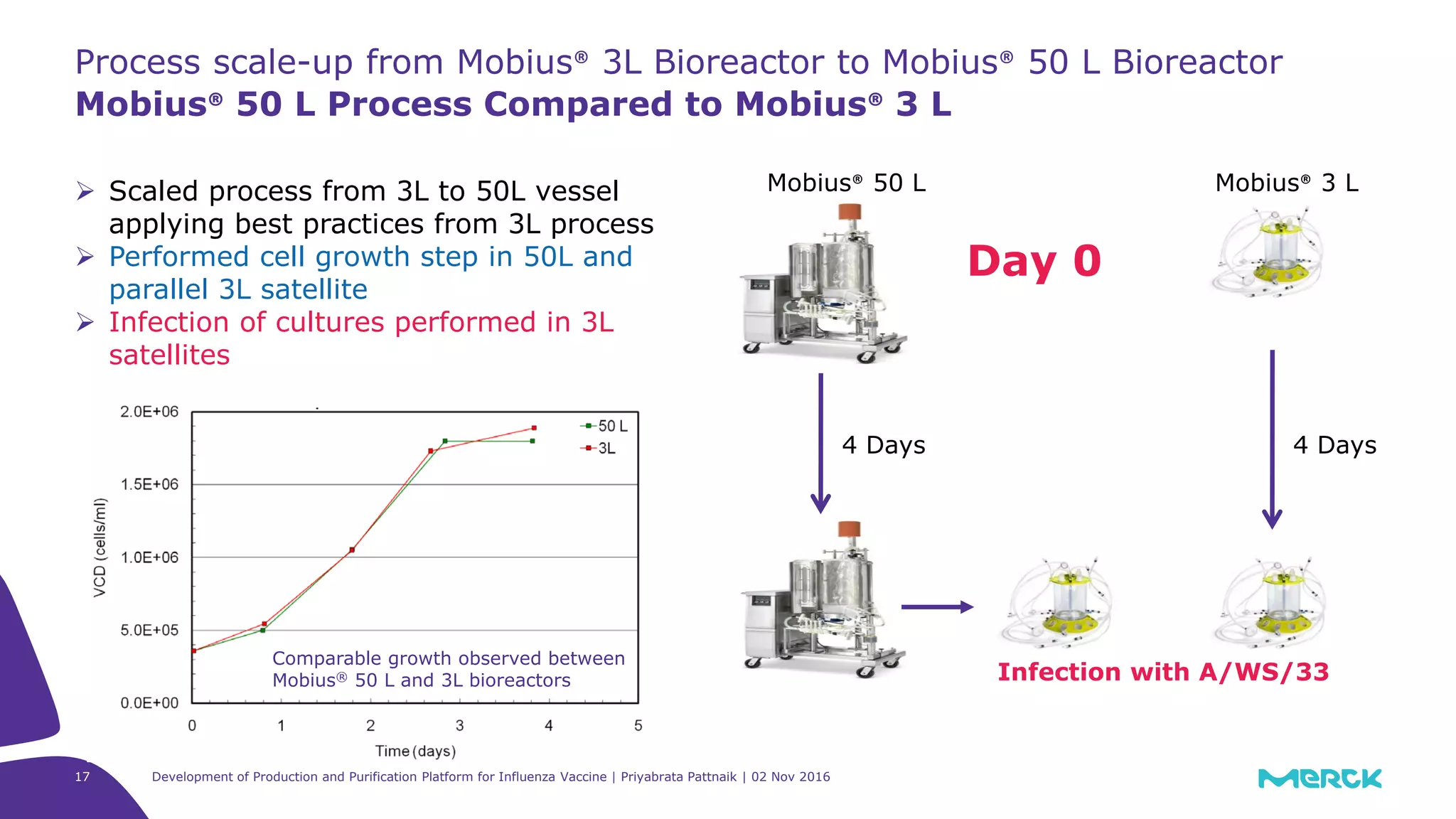

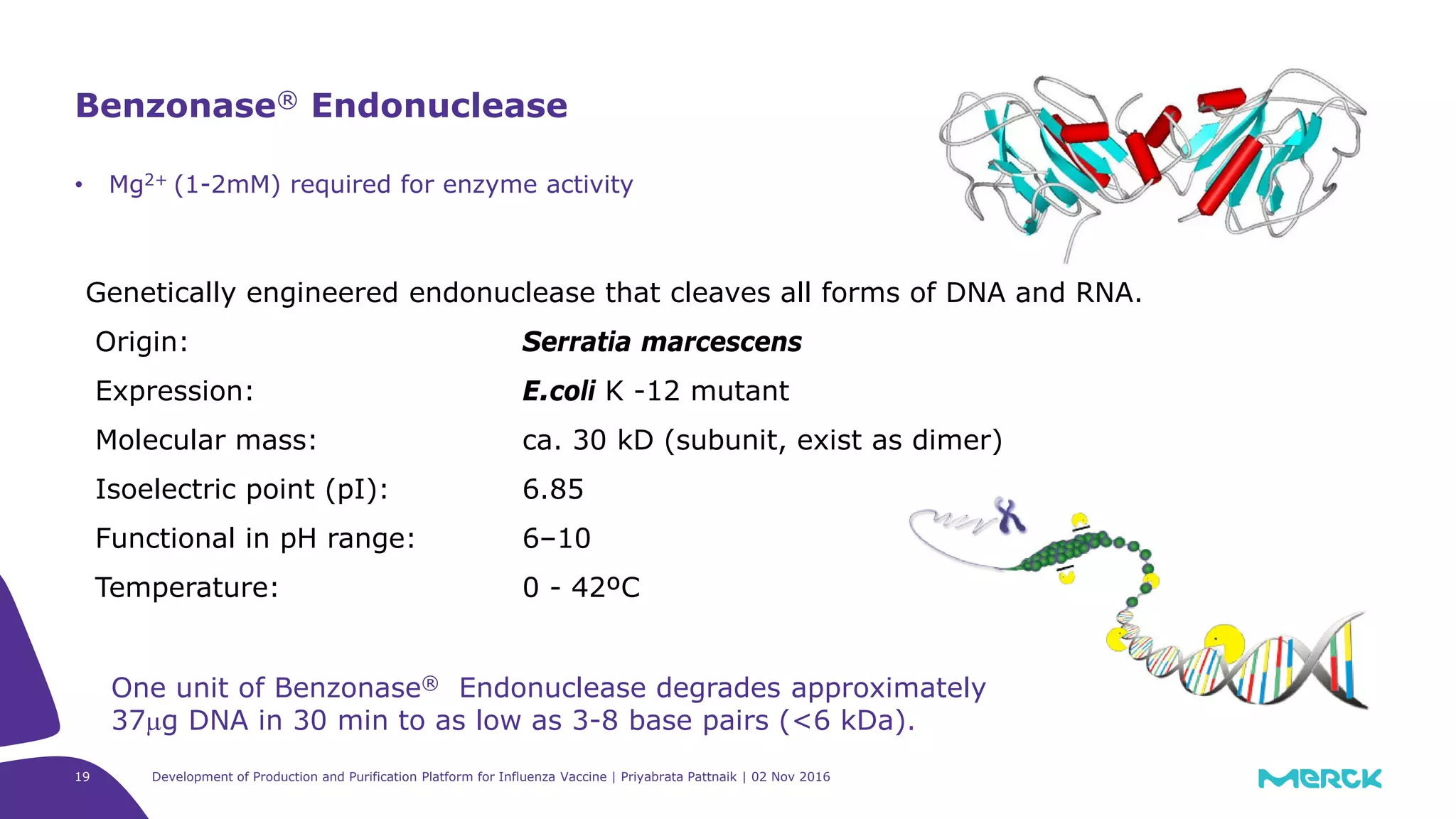
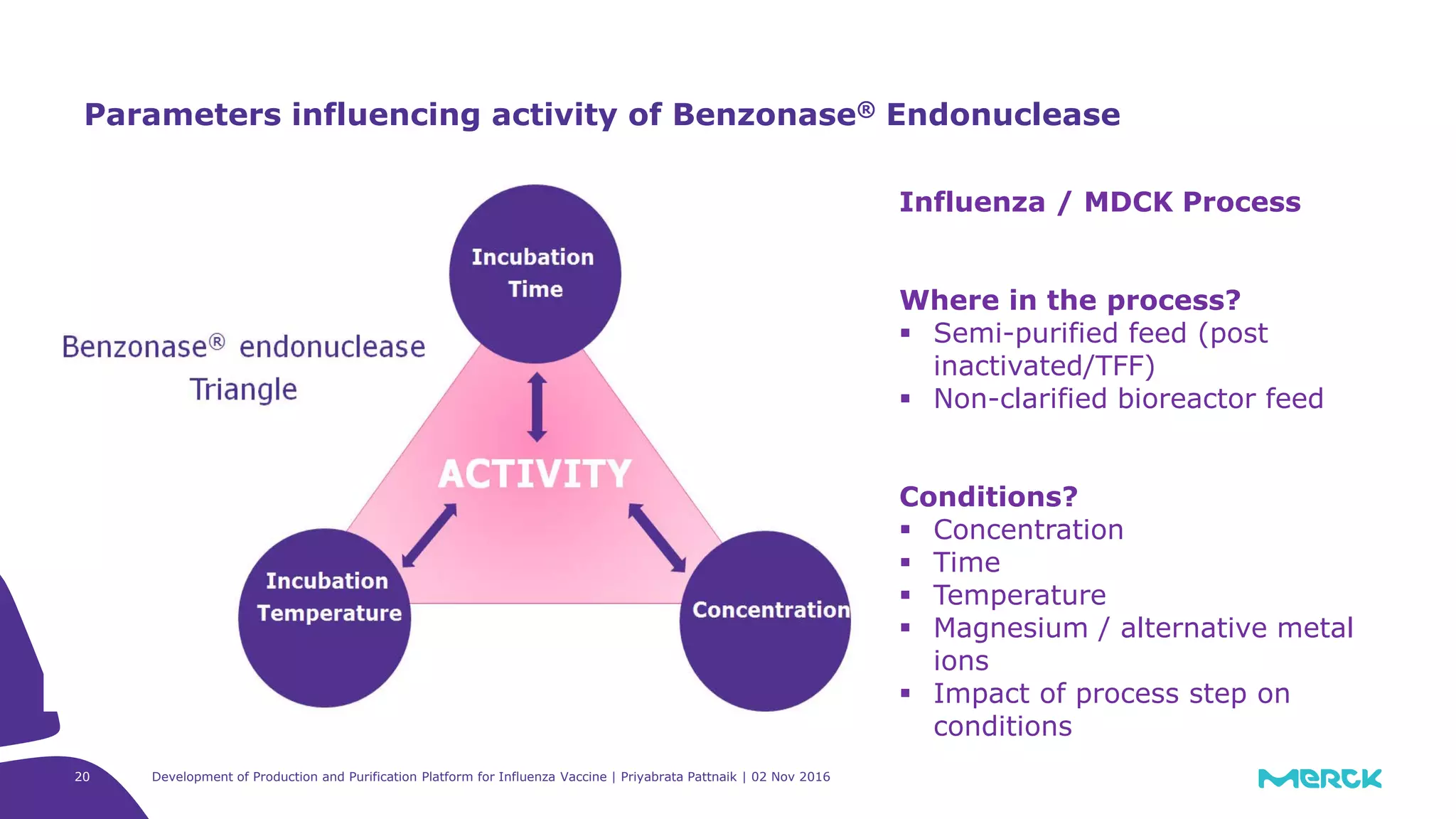
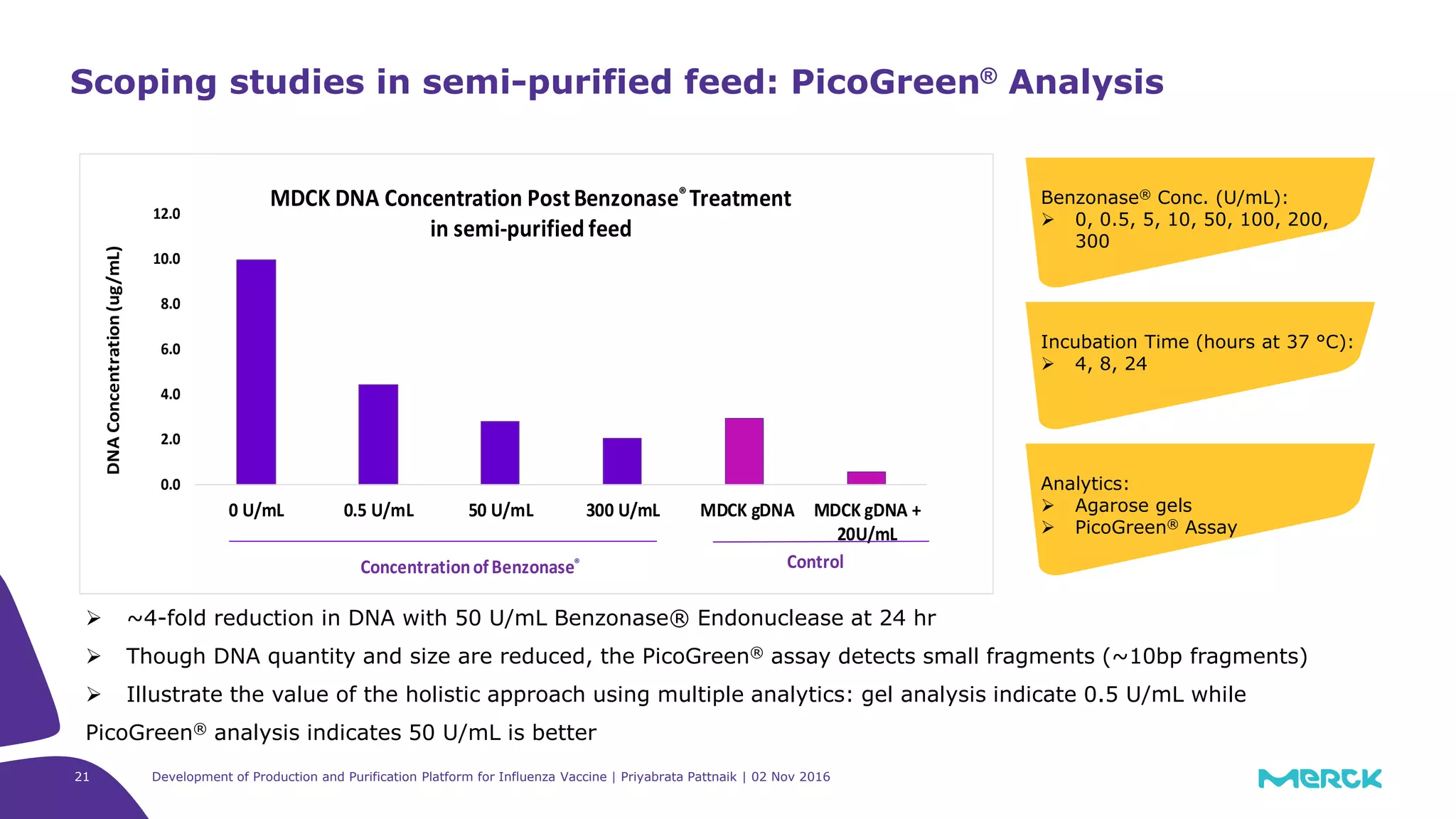
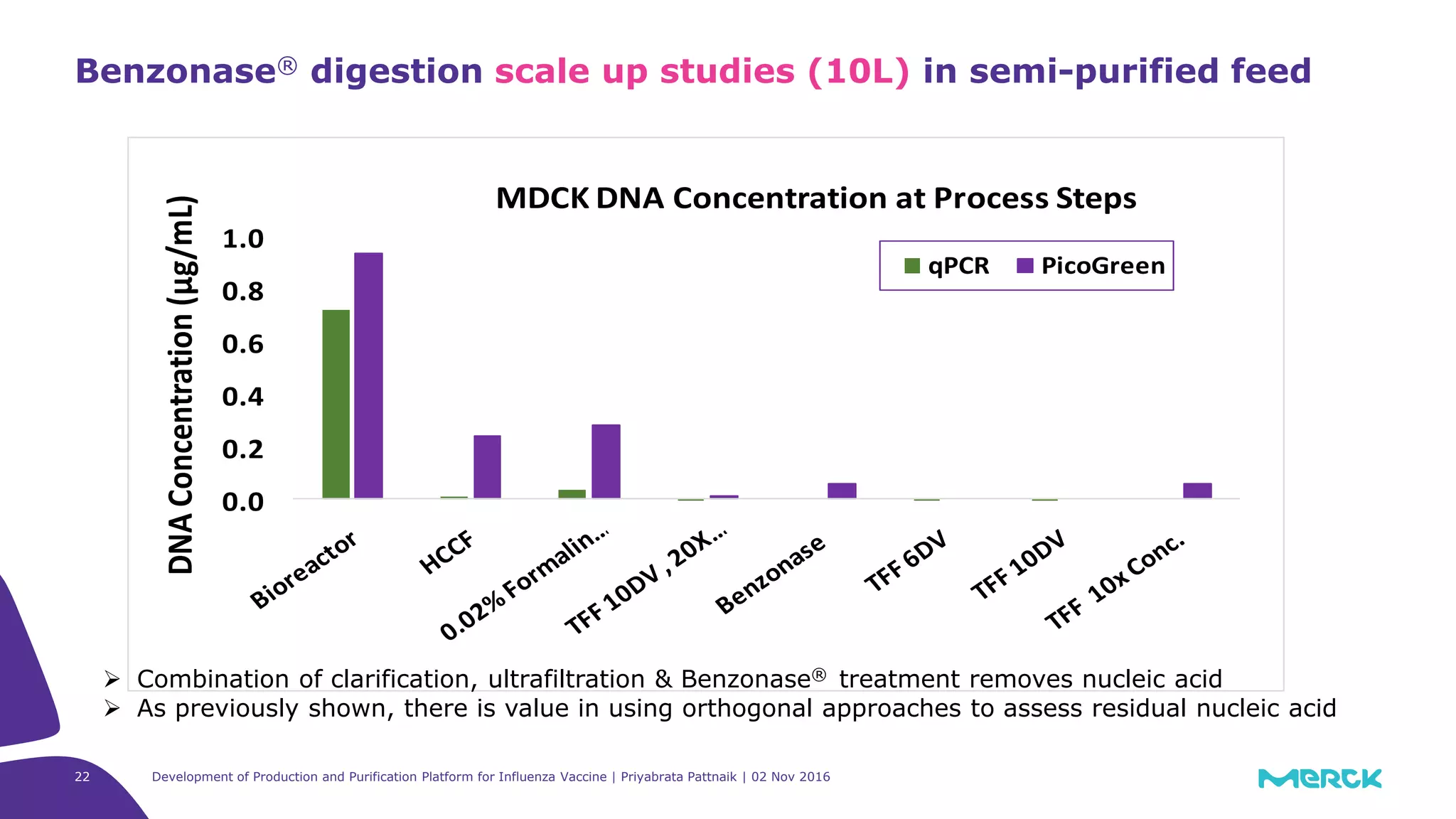
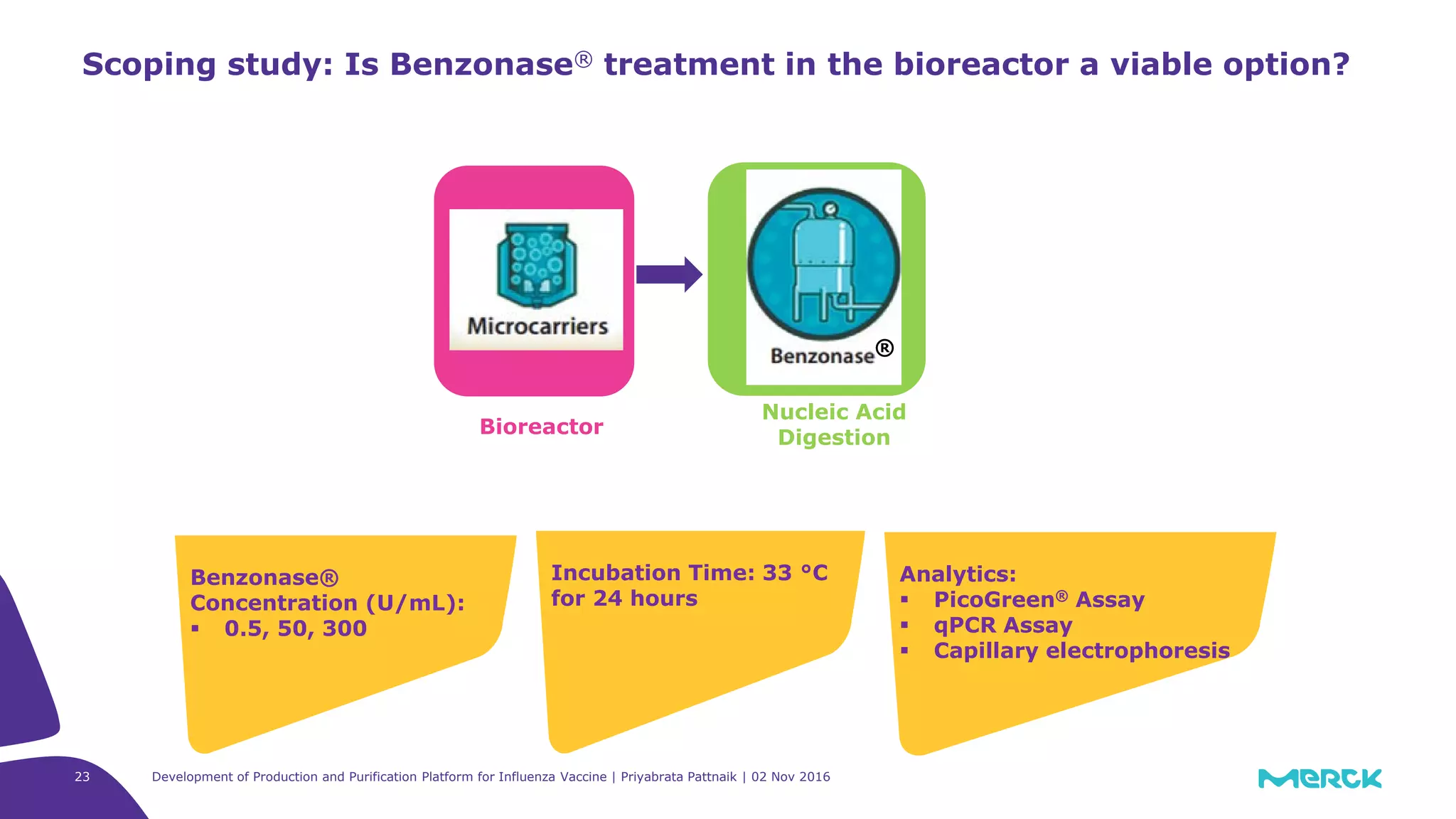
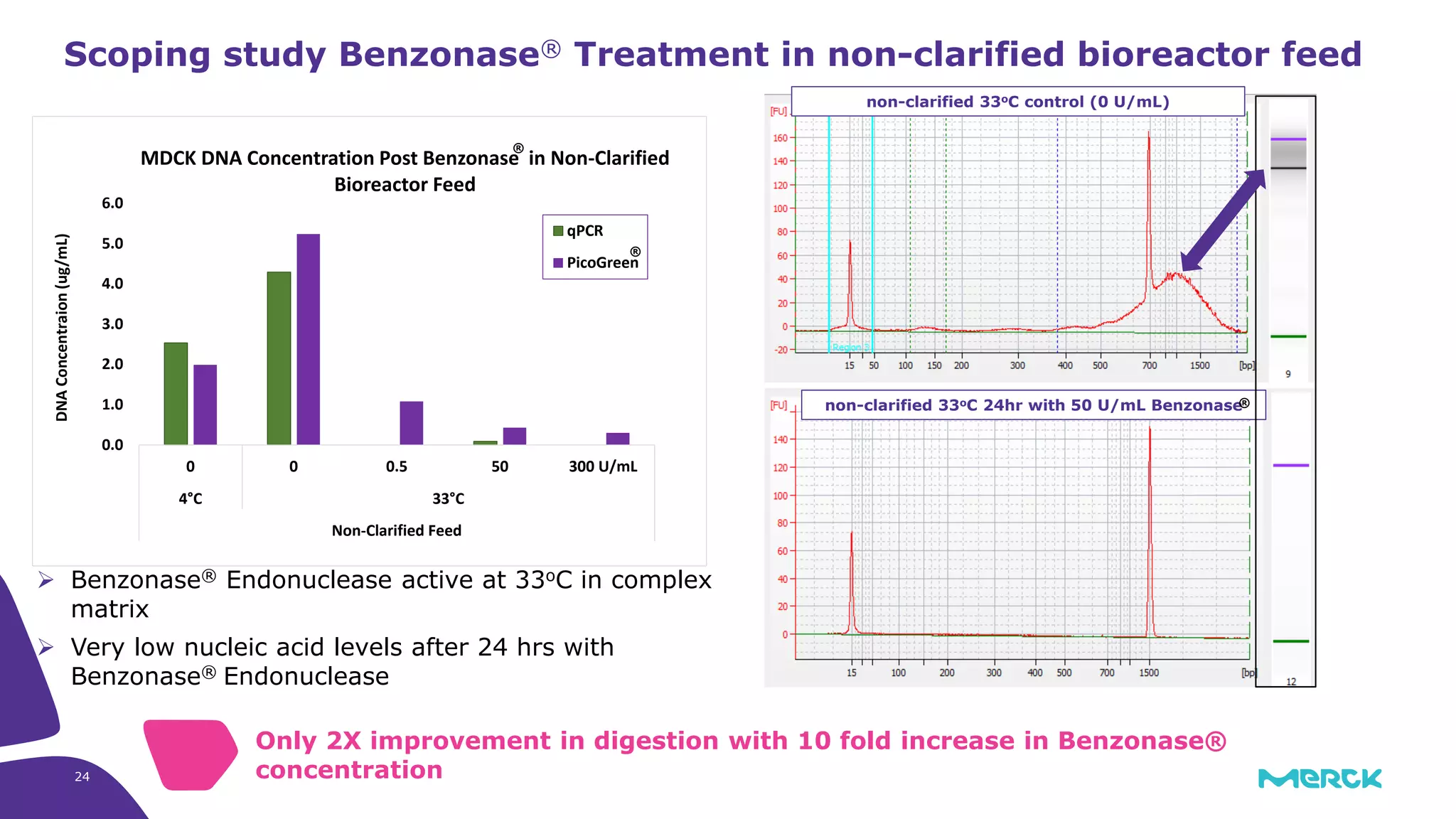

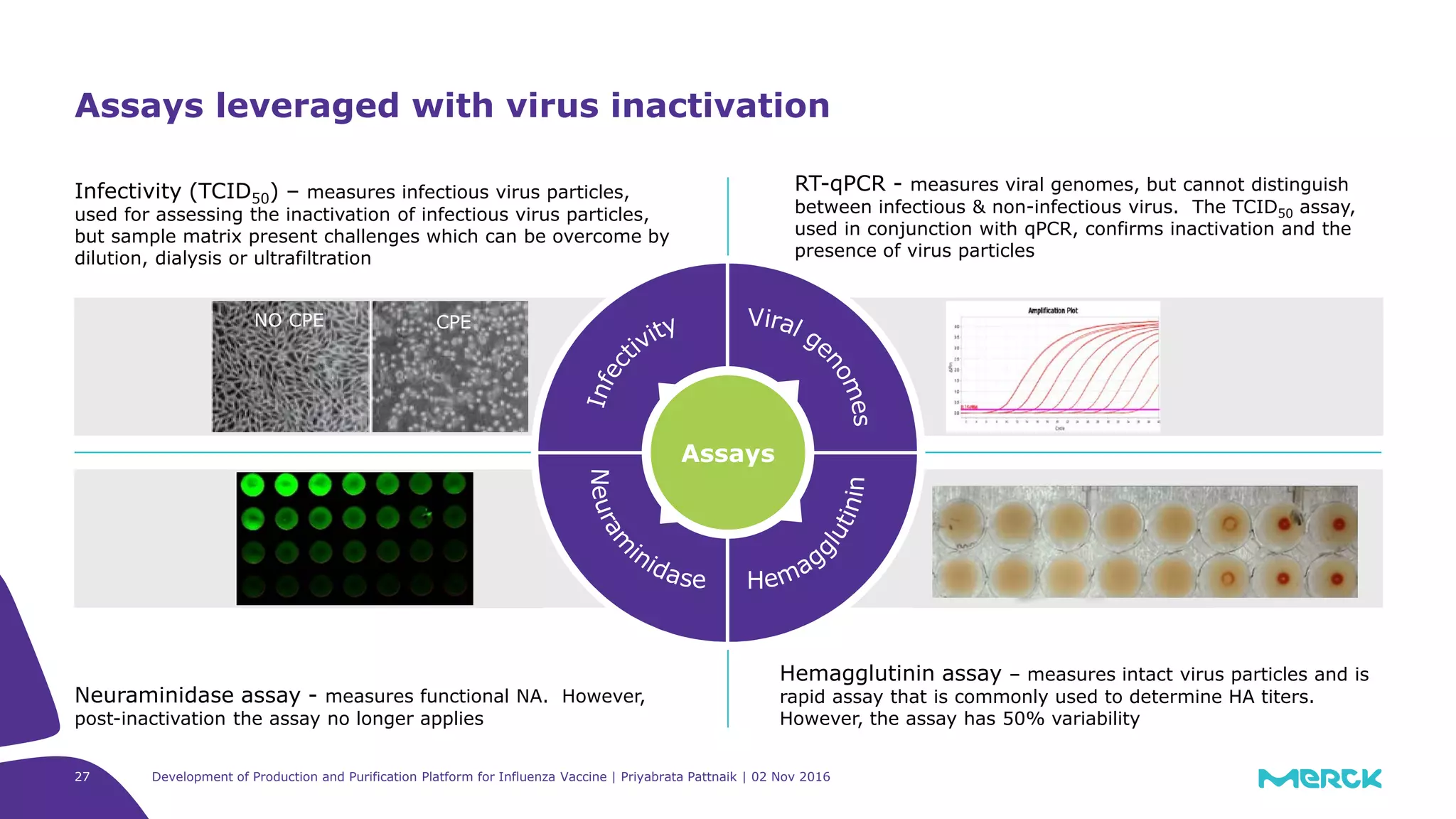
![Full factorial design
3 factors: [formaldehyde], incubation time and
temperature
Varying levels of each factor
5 x 2 x 4 factorial
Experimental setup
Clarified MDCK-based influenza feedstream
Shaker flasks with agitation
Responses
Infectivity assay (TCID50) performed for confirmation
of inactivation of Influenza
Influenza RT-qPCR analysis conducted for presence of
viral genomic RNA before and after inactivation
HA assay performed for hemagglutinin titer (Assay
variability of 50%)
Experimental Design and Responses
Development of Production and Purification Platform for Influenza Vaccine | Priyabrata Pattnaik | 02 Nov 201628
RT = 22°C-23°C](https://image.slidesharecdn.com/devofprodpurificplatformforinfluenzavaccinepattnaik-161115141406/75/Development-of-Production-and-Purification-Platformform-for-Influenza-Vaccine-28-2048.jpg)
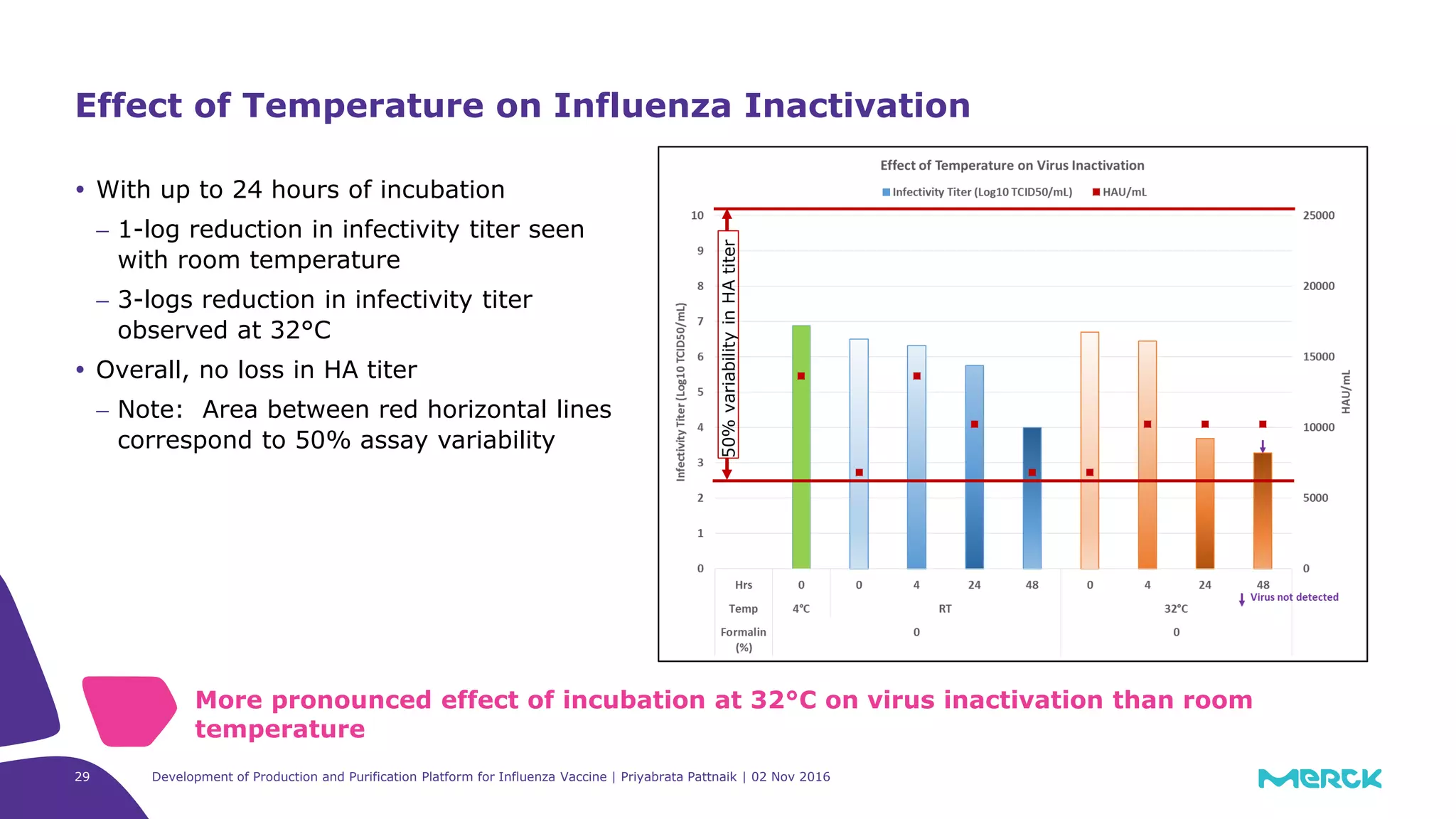
![Development of Production and Purification Platform for Influenza Vaccine | Priyabrata Pattnaik | 02 Nov 201630
5-logs reduction in infectivity titer with
0.02% formaldehyde and 4 hour incubation
at room temperature
No loss in HA titer overall
− Even with increasing [formaldehyde] and
extended incubation time
− Note: Area between red horizontal lines
correspond to 50% assay variability
Effect of Room Temperature and Formaldehyde on Influenza
Inactivation
Influenza inactivation achieved with lowest [formaldehyde] and shortest incubation
time
50%variabilityinHAtiter](https://image.slidesharecdn.com/devofprodpurificplatformforinfluenzavaccinepattnaik-161115141406/75/Development-of-Production-and-Purification-Platformform-for-Influenza-Vaccine-30-2048.jpg)
![Development of Production and Purification Platform for Influenza Vaccine | Priyabrata Pattnaik | 02 Nov 201631
6-logs reduction in infectivity titer with
0.02% formaldehyde and 4 hour incubation
at 32°C
Detrimental effect on HA titer with highest
[formaldehyde] and prolonged incubation
time
− 0.2% formaldehyde for 24 hours
− 0.2% and 0.1% formaldehyde for 48 hours
− Note: Area between red horizontal lines
correspond to 50% assay variability
Effect of 32°C Temperature and Formaldehyde on Influenza
Inactivation
Influenza inactivation achieved with lowest [formaldehyde] and shortest incubation
time
50%variabilityinHAtiter](https://image.slidesharecdn.com/devofprodpurificplatformforinfluenzavaccinepattnaik-161115141406/75/Development-of-Production-and-Purification-Platformform-for-Influenza-Vaccine-31-2048.jpg)