Viruses and vaccine production
•Download as PPTX, PDF•
1 like•236 views
viral vaccine production basics and manufacturing basics involved in development in research. Cell lines and characteristics of cell substrates and mode of operation useful for increased cell density. Basics of vaccine types and their features.
Report
Share
Report
Share
Recommended
Webinar: Re-imagining vaccine manufacturing to address global health challenges

Participate in the interactive on demand webinar: http://bit.ly/ReimaginingVaccinesWebinar
In this webinar, you will learn:
- The evolution of vaccine production in response to pandemics and outbreaks
- Key considerations and perspectives on how vaccine processing and facilities could change to address future global health challenges
COLDCHAIN“Bringing high-quality vaccines and refrigerated medicine to patient...

COLDCHAIN“Bringing high-quality vaccines and refrigerated medicine to patient...Diego Alberto Tamayo
Create a system to track the temperature of vaccine using Smart IoT Edge
devices, Smart IoT cloud Eco Systems, Blockchain and Smart Analytics from
manufacturing to storage to transport to consumption – Reduce Wastage and
Improve distribution and lower Inventory!Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing

Watch the presentation of this webinar here: https://bit.ly/3jmLYHu
State-of-the-art vaccine technologies are transforming vaccine development, and solutions for fast and reliable production are needed.
The vaccine industry has undergone a revolution in technology resulting in a variety of novel therapeutic platforms that accelerate development and significantly reduce the duration for process optimization and scale-up. However, challenges in maintaining efficacy and improving process robustness remain. In this presentation, we present a comparison of these novel technologies, discuss key considerations for manufacturing and share selected case studies for platforms such as virus-like-particles, viral vectors, plasmid DNA, and mRNA platform.
In this webinar, you will learn:
• Benefits of platform technologies in vaccine development
• Key considerations when deciding between platforms
• Vaccine pipeline analysis and selected case studies
Presented by:
David Loong, Ph.D, Senior Consultant, Novel Modalities Asia Pacific, Bioprocessing Strategy
Josephine Cheng, Senior Consultant, Core Modalities Asia Pacific, Bioprocessing Strategy
Ways Forward – Vaccine Manufacturing Tomorrow

In this webinar, you will learn:
Trends in vaccine manufacturing
Innovative solutions in facility design
Case studies and proposals for future vaccine factories
Considerations while setting up Quality Management Systems (QMSs)
How validation helps accelerate regulatory approval
Detailed description:
How we see vaccine manufacturing evolving due to the COVID-19 pandemic, how could it further transform, and what are some solutions we can incorporate to prepare ourselves for next-generation facilities?
The unprecedented COVID-19 pandemic has driven significant tech acceleration around the world, including methods of vaccine manufacturing. Together with the concept of Bioprocessing 4.0, digital biomanufacturing enables centralized orchestration of production process and data management, and a "Facility of the Future" characterized by intensified, continuous, predictive, and autonomous operations. In this presentation, we will explore trends in vaccine manufacturing, including fully single-use processes, closed processing, modular facilities, and platform manufacturing. We will also discuss some key considerations when setting up Quality Management Systems for novel facilities, and how to speed up regulatory approval through best practices in facility validation.
Large-scale Production of Stem Cells Utilizing Microcarriers

Large-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing Microcarriers
Technovax Presentation @ Influenza Congress USA 2012

Looking to venture in the biotech field? Here is our latest "Pitch & Partner Presentation" given on 11/13 in DC and presenting TechnoVax latest projects development and progress.
Recommended
Webinar: Re-imagining vaccine manufacturing to address global health challenges

Participate in the interactive on demand webinar: http://bit.ly/ReimaginingVaccinesWebinar
In this webinar, you will learn:
- The evolution of vaccine production in response to pandemics and outbreaks
- Key considerations and perspectives on how vaccine processing and facilities could change to address future global health challenges
COLDCHAIN“Bringing high-quality vaccines and refrigerated medicine to patient...

COLDCHAIN“Bringing high-quality vaccines and refrigerated medicine to patient...Diego Alberto Tamayo
Create a system to track the temperature of vaccine using Smart IoT Edge
devices, Smart IoT cloud Eco Systems, Blockchain and Smart Analytics from
manufacturing to storage to transport to consumption – Reduce Wastage and
Improve distribution and lower Inventory!Platform Technologies to Accelerate Novel Vaccine Development and Manufacturing

Watch the presentation of this webinar here: https://bit.ly/3jmLYHu
State-of-the-art vaccine technologies are transforming vaccine development, and solutions for fast and reliable production are needed.
The vaccine industry has undergone a revolution in technology resulting in a variety of novel therapeutic platforms that accelerate development and significantly reduce the duration for process optimization and scale-up. However, challenges in maintaining efficacy and improving process robustness remain. In this presentation, we present a comparison of these novel technologies, discuss key considerations for manufacturing and share selected case studies for platforms such as virus-like-particles, viral vectors, plasmid DNA, and mRNA platform.
In this webinar, you will learn:
• Benefits of platform technologies in vaccine development
• Key considerations when deciding between platforms
• Vaccine pipeline analysis and selected case studies
Presented by:
David Loong, Ph.D, Senior Consultant, Novel Modalities Asia Pacific, Bioprocessing Strategy
Josephine Cheng, Senior Consultant, Core Modalities Asia Pacific, Bioprocessing Strategy
Ways Forward – Vaccine Manufacturing Tomorrow

In this webinar, you will learn:
Trends in vaccine manufacturing
Innovative solutions in facility design
Case studies and proposals for future vaccine factories
Considerations while setting up Quality Management Systems (QMSs)
How validation helps accelerate regulatory approval
Detailed description:
How we see vaccine manufacturing evolving due to the COVID-19 pandemic, how could it further transform, and what are some solutions we can incorporate to prepare ourselves for next-generation facilities?
The unprecedented COVID-19 pandemic has driven significant tech acceleration around the world, including methods of vaccine manufacturing. Together with the concept of Bioprocessing 4.0, digital biomanufacturing enables centralized orchestration of production process and data management, and a "Facility of the Future" characterized by intensified, continuous, predictive, and autonomous operations. In this presentation, we will explore trends in vaccine manufacturing, including fully single-use processes, closed processing, modular facilities, and platform manufacturing. We will also discuss some key considerations when setting up Quality Management Systems for novel facilities, and how to speed up regulatory approval through best practices in facility validation.
Large-scale Production of Stem Cells Utilizing Microcarriers

Large-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing MicrocarriersLarge-scale Production of Stem Cells Utilizing Microcarriers
Technovax Presentation @ Influenza Congress USA 2012

Looking to venture in the biotech field? Here is our latest "Pitch & Partner Presentation" given on 11/13 in DC and presenting TechnoVax latest projects development and progress.
Bridging The Valley Of Death A Tale Of Two Cultures

A brief presentation for the upcoming Bioconference Live meeting
Ebola vaccine market analysis

A brief presentation of the market space created by the ongoing ebola epidemic, and projected cost estimates for scale up production vaccine introduction of an ebola vaccine.
Biosafety Testing Solutions for Cell & Gene Therapy

Abstract:
Cell and gene therapies, well recognized as the drug revolution for this decade, are booming in Asian countries. Several cell and gene therapeutic products launched successfully in Europe and the US. The commercialization of these therapies is a hot topic, while ensuring product safety, especially quality for the new modalities, raises challenges within the industry. As a globally leading biosafety testing provider, Merck is committed to optimizing and advancing innovation and development of biosafety testing. As your reliable partner in CMC consideration, our comprehensive solutions for cell and gene therapy biosafety testing enable regulatory compliance. This presentation will cover rationale and methodologies for cell and gene therapy product testing from Merck’s BioReliance® testing portfolio, as well as provide an overview of our testing capabilities and services.
Development and strategies of cell-culture technology for influenza vaccine [...

Development and strategies of cell-culture technology for influenza vaccine [Applied biotechnology]
Production and Purification of Virus Like Particle (VLP) based Vaccine

Presentation at IBC's Bioprocess & Technology Conference, 27-30 October 2015, Singapore
Sartorius Solutions for Cell & Gene based Therapies

Sartorius is a well respected global solution provider within the biologics industry, especially for antibody and vaccine production. Our proven products and services are being diversified for upstream and downstream processing of cells and viruses for allogeneic and autologous advanced therapies.
Sartorius provides the cellular immunotherapy industry with a range of scalable single-use production technologies. Our portfolio supports viral vector transduction, cell expansion, and downstream processing steps including harvest, wash, and concentration of cells
Virus Safety in Vaccine Production

Presentation at DCVMN & Merck Advanced Workshop on "Bioprocess Optimization" at Singapore, 7-8 Feb 2018.
Developing a single use adenovirus-vectored vaccine process through public-pr...

This work highlights the importance of collaborations to accelerate vaccine process development and manufacturing under the constant pressure of emerging diseases and the growing need of global immunizations.
We are collaborating with the Jenner Institute of the University of Oxford to advance the development of a rapid, scalable and GMP compliant process for simian adenoviruses used as vector for vaccines such as Rabies and emerging threats like Zika and Ebola. This webinar will describe the transition from a labor and time intensive process development to one utilizing a maximum of disposable technologies such as single use bioreactors and filtration technologies, using the rabies vaccine as a first candidate. We will highlight the challenges and their corresponding solutions that in the end created a template that can be used for different types of adenoviral vectors-based vaccines manufacturing.
In this webinar, you will learn:
- The challenges of creating a rapid and scalable process for Adenovirus vector manufacturing.
- The solutions that overcame those challenges.
- How public-private collaborations can accelerate vaccine process development.
Virus like-particles-based-vaccine-development-services

Virus-like Particles (VLPs) Based Vaccine, an approach that fights viruses with its own weapon, is one of the most exciting emerging vaccine technologies for generating effective and long-lasting protection. https://www.creative-biolabs.com/vaccine/virus-like-particles-based-vaccines.htm
Production and purification of Viral vectors for gene and cell therapy appli...

Production and purification of Viral vectors for gene and cell therapy appli...Dr. Priyabrata Pattnaik
Presentation at "2016 Osong BioExcellence - Renaissance in Immunotherapy" at South Korea, an event jointly hosted by Kbio Health and Merck on 6th October 2016. Emerging Viral Risks and Mitigation Strategies in Biologics Manufacturing

Emerging viruses represent a constant challenge to biopharmaceutical manufacturers, and therefore formal risk assessments and informed programs of safety testing are necessary to assure safety. Emerging viruses such as the Zika virus have the potential to contaminate raw materials of human origin, Schmallenberg virus is a contaminant of bovine serum, and the long-known, but often ignored, Hepatitis E virus represents further challenges to the safety of raw materials. Results of in vitro culture and molecular testing strategies of raw materials for viruses with diverse characteristics will be presented, and holistic approaches to mitigate the risk of novel viruses to the safety of raw materials will be outlined.
In this webinar, you will learn:
-The identity of emerging viruses and potential impact on the safety of raw materials and final products
-Testing strategies for specific viruses
-Holistic approaches to mitigate the risk of novel viruses in raw materials
Achieving High Yields in Scalable Xeno Free Culture Formats with Mesenchymal ...

Watch the presentation of this webinar here: https://bit.ly/3ryE5ST
Optimize your mesenchymal stem cell growth. Join our webinar to learn more about our GMP-compliant xeno free media formulation that supports high performance expansions and compatibility with scalable xeno free manufacturing conditions.
Optimizing ex vivo cell expansion processes in preparation for clinical use is a critical step in cell therapy manufacturing. Given the curative and lifesaving impacts these therapies can have on patients, overcoming roadblocks with scalability and supply chain, using high quality raw materials are essential for therapeutic access.
The GMP-compliant Stemline® XF MSC Medium and cocktail promotes expansion of human mesenchymal stromal/stem cells (hMSCs) to high densities while maintaining cell identity and quality. This product was designed for derivation and expansion of MSCs using xeno free conditions in planar and microcarrier-based culture platforms, easing the transfer between research, clinical, and manufacturing scale cultures.
In this webinar, you will:
• Explore the current landscape and future trends of cell culture media for adult mesenchymal stem cells
• Discover ways to derive MSC's from Bone Marrow in Xeno Free conditions from static to microcarrier-based suspension culture platforms.
• Learn how Stemline® XF MSC Media provides robust performance and reduces scalability roadblocks
Presented by: Kathleen Ongena, Ph.D., Head of Customer Applications and Mark Ventresco, Cell Therapy Product Manager
Accelerating vaccine development and manufacturing

Oral presentation in VacChina - The 4th Annual Vaccine Development Summit, 08-09 April, 2014, Beijing, China.
Poultry India - Knowledge Day 2015 Speaker Dr. Marcelo Paniago

Poultry India 2015 - Knowledge Day Technical Seminar - Presentation by Dr. Marcelo Paniago on "Emerging Innovation in Vaccinology"
Applications of cell culture

This presentation is about various applications of cell cultures in human/animal viral vaccine production.
new generation vaccine.pptx

vaccine train user immune system to create antibodies, just as it when it is exposed to a disease. However, because vaccine contain only killed or weakened forms of germs like viruses or bacteria, they do not cause the disease or put you at the risk of complications.
vaccine is a biological preparation that improve immunity to a particular disease.
A vaccine typically contain an agent that resembles a disease causing microorganisms and is often made from weakened or killed forms of the microbes.
More Related Content
What's hot
Bridging The Valley Of Death A Tale Of Two Cultures

A brief presentation for the upcoming Bioconference Live meeting
Ebola vaccine market analysis

A brief presentation of the market space created by the ongoing ebola epidemic, and projected cost estimates for scale up production vaccine introduction of an ebola vaccine.
Biosafety Testing Solutions for Cell & Gene Therapy

Abstract:
Cell and gene therapies, well recognized as the drug revolution for this decade, are booming in Asian countries. Several cell and gene therapeutic products launched successfully in Europe and the US. The commercialization of these therapies is a hot topic, while ensuring product safety, especially quality for the new modalities, raises challenges within the industry. As a globally leading biosafety testing provider, Merck is committed to optimizing and advancing innovation and development of biosafety testing. As your reliable partner in CMC consideration, our comprehensive solutions for cell and gene therapy biosafety testing enable regulatory compliance. This presentation will cover rationale and methodologies for cell and gene therapy product testing from Merck’s BioReliance® testing portfolio, as well as provide an overview of our testing capabilities and services.
Development and strategies of cell-culture technology for influenza vaccine [...

Development and strategies of cell-culture technology for influenza vaccine [Applied biotechnology]
Production and Purification of Virus Like Particle (VLP) based Vaccine

Presentation at IBC's Bioprocess & Technology Conference, 27-30 October 2015, Singapore
Sartorius Solutions for Cell & Gene based Therapies

Sartorius is a well respected global solution provider within the biologics industry, especially for antibody and vaccine production. Our proven products and services are being diversified for upstream and downstream processing of cells and viruses for allogeneic and autologous advanced therapies.
Sartorius provides the cellular immunotherapy industry with a range of scalable single-use production technologies. Our portfolio supports viral vector transduction, cell expansion, and downstream processing steps including harvest, wash, and concentration of cells
Virus Safety in Vaccine Production

Presentation at DCVMN & Merck Advanced Workshop on "Bioprocess Optimization" at Singapore, 7-8 Feb 2018.
Developing a single use adenovirus-vectored vaccine process through public-pr...

This work highlights the importance of collaborations to accelerate vaccine process development and manufacturing under the constant pressure of emerging diseases and the growing need of global immunizations.
We are collaborating with the Jenner Institute of the University of Oxford to advance the development of a rapid, scalable and GMP compliant process for simian adenoviruses used as vector for vaccines such as Rabies and emerging threats like Zika and Ebola. This webinar will describe the transition from a labor and time intensive process development to one utilizing a maximum of disposable technologies such as single use bioreactors and filtration technologies, using the rabies vaccine as a first candidate. We will highlight the challenges and their corresponding solutions that in the end created a template that can be used for different types of adenoviral vectors-based vaccines manufacturing.
In this webinar, you will learn:
- The challenges of creating a rapid and scalable process for Adenovirus vector manufacturing.
- The solutions that overcame those challenges.
- How public-private collaborations can accelerate vaccine process development.
Virus like-particles-based-vaccine-development-services

Virus-like Particles (VLPs) Based Vaccine, an approach that fights viruses with its own weapon, is one of the most exciting emerging vaccine technologies for generating effective and long-lasting protection. https://www.creative-biolabs.com/vaccine/virus-like-particles-based-vaccines.htm
Production and purification of Viral vectors for gene and cell therapy appli...

Production and purification of Viral vectors for gene and cell therapy appli...Dr. Priyabrata Pattnaik
Presentation at "2016 Osong BioExcellence - Renaissance in Immunotherapy" at South Korea, an event jointly hosted by Kbio Health and Merck on 6th October 2016. Emerging Viral Risks and Mitigation Strategies in Biologics Manufacturing

Emerging viruses represent a constant challenge to biopharmaceutical manufacturers, and therefore formal risk assessments and informed programs of safety testing are necessary to assure safety. Emerging viruses such as the Zika virus have the potential to contaminate raw materials of human origin, Schmallenberg virus is a contaminant of bovine serum, and the long-known, but often ignored, Hepatitis E virus represents further challenges to the safety of raw materials. Results of in vitro culture and molecular testing strategies of raw materials for viruses with diverse characteristics will be presented, and holistic approaches to mitigate the risk of novel viruses to the safety of raw materials will be outlined.
In this webinar, you will learn:
-The identity of emerging viruses and potential impact on the safety of raw materials and final products
-Testing strategies for specific viruses
-Holistic approaches to mitigate the risk of novel viruses in raw materials
Achieving High Yields in Scalable Xeno Free Culture Formats with Mesenchymal ...

Watch the presentation of this webinar here: https://bit.ly/3ryE5ST
Optimize your mesenchymal stem cell growth. Join our webinar to learn more about our GMP-compliant xeno free media formulation that supports high performance expansions and compatibility with scalable xeno free manufacturing conditions.
Optimizing ex vivo cell expansion processes in preparation for clinical use is a critical step in cell therapy manufacturing. Given the curative and lifesaving impacts these therapies can have on patients, overcoming roadblocks with scalability and supply chain, using high quality raw materials are essential for therapeutic access.
The GMP-compliant Stemline® XF MSC Medium and cocktail promotes expansion of human mesenchymal stromal/stem cells (hMSCs) to high densities while maintaining cell identity and quality. This product was designed for derivation and expansion of MSCs using xeno free conditions in planar and microcarrier-based culture platforms, easing the transfer between research, clinical, and manufacturing scale cultures.
In this webinar, you will:
• Explore the current landscape and future trends of cell culture media for adult mesenchymal stem cells
• Discover ways to derive MSC's from Bone Marrow in Xeno Free conditions from static to microcarrier-based suspension culture platforms.
• Learn how Stemline® XF MSC Media provides robust performance and reduces scalability roadblocks
Presented by: Kathleen Ongena, Ph.D., Head of Customer Applications and Mark Ventresco, Cell Therapy Product Manager
Accelerating vaccine development and manufacturing

Oral presentation in VacChina - The 4th Annual Vaccine Development Summit, 08-09 April, 2014, Beijing, China.
Poultry India - Knowledge Day 2015 Speaker Dr. Marcelo Paniago

Poultry India 2015 - Knowledge Day Technical Seminar - Presentation by Dr. Marcelo Paniago on "Emerging Innovation in Vaccinology"
What's hot (18)
Bridging The Valley Of Death A Tale Of Two Cultures

Bridging The Valley Of Death A Tale Of Two Cultures
Biosafety Testing Solutions for Cell & Gene Therapy

Biosafety Testing Solutions for Cell & Gene Therapy
Development and strategies of cell-culture technology for influenza vaccine [...

Development and strategies of cell-culture technology for influenza vaccine [...
VERO Bioreactor Cell Culture and Vaccine Production 4TR1- Capstone Project

VERO Bioreactor Cell Culture and Vaccine Production 4TR1- Capstone Project
Production and Purification of Virus Like Particle (VLP) based Vaccine

Production and Purification of Virus Like Particle (VLP) based Vaccine
Sartorius Solutions for Cell & Gene based Therapies

Sartorius Solutions for Cell & Gene based Therapies
Developing a single use adenovirus-vectored vaccine process through public-pr...

Developing a single use adenovirus-vectored vaccine process through public-pr...
Virus like-particles-based-vaccine-development-services

Virus like-particles-based-vaccine-development-services
Production and purification of Viral vectors for gene and cell therapy appli...

Production and purification of Viral vectors for gene and cell therapy appli...
Emerging Viral Risks and Mitigation Strategies in Biologics Manufacturing

Emerging Viral Risks and Mitigation Strategies in Biologics Manufacturing
Achieving High Yields in Scalable Xeno Free Culture Formats with Mesenchymal ...

Achieving High Yields in Scalable Xeno Free Culture Formats with Mesenchymal ...
Accelerating vaccine development and manufacturing

Accelerating vaccine development and manufacturing
Poultry India - Knowledge Day 2015 Speaker Dr. Marcelo Paniago

Poultry India - Knowledge Day 2015 Speaker Dr. Marcelo Paniago
Similar to Viruses and vaccine production
Applications of cell culture

This presentation is about various applications of cell cultures in human/animal viral vaccine production.
new generation vaccine.pptx

vaccine train user immune system to create antibodies, just as it when it is exposed to a disease. However, because vaccine contain only killed or weakened forms of germs like viruses or bacteria, they do not cause the disease or put you at the risk of complications.
vaccine is a biological preparation that improve immunity to a particular disease.
A vaccine typically contain an agent that resembles a disease causing microorganisms and is often made from weakened or killed forms of the microbes.
new generation vaccine.pptx

Immunity: Protection from an infectious disease. If you are immune to a disease, you can be exposed to it without becoming infected.
Vaccine: A preparation that is used to stimulate the body’s immune response against diseases. Vaccines are usually administered through needle injections, but some can be administered by mouth or sprayed into the nose.
Vaccination: The act of introducing a vaccine into the body to produce protection from a specific disease.
Mechanism of different types of vaccines in development

Recap of certain vaccines technologies against Covid-19
Introduce MOA of current and in development Covid-19 vaccines
Ever since the first vaccine was developed in 1796 to treat smallpox, several different methods have been created to develop successful vaccines. Today, those methods, known as vaccine technologies, are more advanced and use the latest technology to help protect the world from preventable diseases.
Depending on the pathogen (a bacteria or virus) that is being targeted, different vaccine technologies are used to generate an effective vaccine.
In total, there are five different vaccine technology platforms in this presentation each with its own benefits, and examples.
Vero cells for vaccine production

Compiled by Nagendra P and Pritam Vishu Bagwe
M.Tech Pharmaceutical Sciences
Department of Pharmaceutical Sciences and Technology
Institute of Chemical Technology, Matunga, Mumbai, India.
Vero cells are the continuous cell lines which is employed in the production of viral vaccines . This cell line has the ability to be scaled up and grown in large bioreactors using microcarrier beads .
Viral vaccine production (5 MIN READ)

Production of vaccine industrially explained in 5 min. Factors under consideration and storage. Types of vaccine produced all under one presentation.
Impact of biotechnology

Most developments in biotechnology originated for their potential applications in health care.
Contributions of biotechnology are more frequent, more notable and more rewarding in health sector.
Vaccination

Vaccine, Types of vaccine, Process of Vaccine, Methods of the different vaccines. Reading and learn all this thing vaccine production helps to make a bright career in life science.
Cultivation Of Viruses -Methods and Conditions

It is about various methods for cultivation of viruses, how to isolate them and why to isolate them.
Similar to Viruses and vaccine production (20)
Mechanism of different types of vaccines in development

Mechanism of different types of vaccines in development
More from Institute of chemical Technology, Mumbai
Probiotics and benefits

Probiotics are live microorganisms that confer health benefits when colonize the gastrointestinal tract. The various microbial strains are now found to provide therapeutic effects through the metabolites they produce, digestion of dietary fibers, inhibition of pathogen adhesion, provide missing enzyme, maintaining homeostasis and also controlling brain activities which may lead to autism if disturbed.
Bioassays for protein, Vitamins and Antibiotics

Bioassays are assays or biological techniques to measure strength, potency, concentration or efficacy of any substance by its effect on biological substance like tissues, cells, animals or enzymes etc
Critical Review (drug toxicity testing using organ on chip)

Here you will know , how to write a critical review & what sections are suppose to analyse why reading a paper. Here is a critical review of a paper titled ' Repeated dose multi-drug testing using a microfluidic chip-based
coculture of human liver and kidney proximal tubules equivalents' published recently in 2020 in reputed journal nature.
Superoxide dismutase

Superoxide dismutase is an enzyme that helps break down potentially harmful oxygen molecules in cells. This might prevent damage to tissues. It is being researched to see if it can help conditions where harmful oxygen molecules are believed to play a role in disease.
Asparaginase : Importance and production 

Asparaginase is an important enzyme in Medicine & food industry. It catalyzes Asparagine to aspartate and Ammonia. The purpose of using asparaginase in foods is to reduce the levels of acrylamide that form in certain carbohydrate-rich foods during cooking.The rationale behind asparaginase is that it takes advantage of the fact that acute lymphoblastic leukemia cells and some other suspected tumor cells are unable to synthesize the non-essential amino acid asparagine, whereas normal cells are able to make their own asparagine.
predicting promotor strength 

summary and comparison of two papers revealing strength of promotor sequences through bioinformatics and invitro assay.
PHI (promotor Homology index) is one way to quantify strength of promotors while RNA expression profiling by micro-array is another way to predict strength by fluorescent intensity measured through this assay.
Hydrophobic Interaction Chromatography (Monoclonal Antibody purification with...

Hydrophobic Interaction Chromatography (Monoclonal Antibody purification with...Institute of chemical Technology, Mumbai
HIC (Hydrophobic Interaction Chromatography) is used to purify Abs (Antibodies) by conventional procedure. This presentation gives a brief about non-conventional mode of HIC process operation for optimization of conditions like ligand nature, mobile phase pH, column loading, product selectivity and more to avoid harsh nature of salts like Ammonium sulfate.Disposable bioreactors VS stainless steel bioreactors

Disposable bioreactors are gaining popularity in Bioprocess industry and biopharmaceutical manufacturing.
Detection of Babesia canis by PCR assay

Detection of Parasite DNA by using PCR and suitable primers.
Polymerase Chain Reaction
Bones and cartilages tissue engineering

tissue engineering basics for bone & cartilage and Biomaterials
More from Institute of chemical Technology, Mumbai (11)
Critical Review (drug toxicity testing using organ on chip)

Critical Review (drug toxicity testing using organ on chip)
Hydrophobic Interaction Chromatography (Monoclonal Antibody purification with...

Hydrophobic Interaction Chromatography (Monoclonal Antibody purification with...
Disposable bioreactors VS stainless steel bioreactors

Disposable bioreactors VS stainless steel bioreactors
Recently uploaded
Ocular injury ppt Upendra pal optometrist upums saifai etawah

This ppt are composed by upendra pal to provide help future optometrist of india
NVBDCP.pptx Nation vector borne disease control program

NVBDCP was launched in 2003-2004 . Vector-Borne Disease: Disease that results from an infection transmitted to humans and other animals by blood-feeding arthropods, such as mosquitoes, ticks, and fleas. Examples of vector-borne diseases include Dengue fever, West Nile Virus, Lyme disease, and malaria.
Physiology of Chemical Sensation of smell.pdf

Title: Sense of Smell
Presenter: Dr. Faiza, Assistant Professor of Physiology
Qualifications:
MBBS (Best Graduate, AIMC Lahore)
FCPS Physiology
ICMT, CHPE, DHPE (STMU)
MPH (GC University, Faisalabad)
MBA (Virtual University of Pakistan)
Learning Objectives:
Describe the primary categories of smells and the concept of odor blindness.
Explain the structure and location of the olfactory membrane and mucosa, including the types and roles of cells involved in olfaction.
Describe the pathway and mechanisms of olfactory signal transmission from the olfactory receptors to the brain.
Illustrate the biochemical cascade triggered by odorant binding to olfactory receptors, including the role of G-proteins and second messengers in generating an action potential.
Identify different types of olfactory disorders such as anosmia, hyposmia, hyperosmia, and dysosmia, including their potential causes.
Key Topics:
Olfactory Genes:
3% of the human genome accounts for olfactory genes.
400 genes for odorant receptors.
Olfactory Membrane:
Located in the superior part of the nasal cavity.
Medially: Folds downward along the superior septum.
Laterally: Folds over the superior turbinate and upper surface of the middle turbinate.
Total surface area: 5-10 square centimeters.
Olfactory Mucosa:
Olfactory Cells: Bipolar nerve cells derived from the CNS (100 million), with 4-25 olfactory cilia per cell.
Sustentacular Cells: Produce mucus and maintain ionic and molecular environment.
Basal Cells: Replace worn-out olfactory cells with an average lifespan of 1-2 months.
Bowman’s Gland: Secretes mucus.
Stimulation of Olfactory Cells:
Odorant dissolves in mucus and attaches to receptors on olfactory cilia.
Involves a cascade effect through G-proteins and second messengers, leading to depolarization and action potential generation in the olfactory nerve.
Quality of a Good Odorant:
Small (3-20 Carbon atoms), volatile, water-soluble, and lipid-soluble.
Facilitated by odorant-binding proteins in mucus.
Membrane Potential and Action Potential:
Resting membrane potential: -55mV.
Action potential frequency in the olfactory nerve increases with odorant strength.
Adaptation Towards the Sense of Smell:
Rapid adaptation within the first second, with further slow adaptation.
Psychological adaptation greater than receptor adaptation, involving feedback inhibition from the central nervous system.
Primary Sensations of Smell:
Camphoraceous, Musky, Floral, Pepperminty, Ethereal, Pungent, Putrid.
Odor Detection Threshold:
Examples: Hydrogen sulfide (0.0005 ppm), Methyl-mercaptan (0.002 ppm).
Some toxic substances are odorless at lethal concentrations.
Characteristics of Smell:
Odor blindness for single substances due to lack of appropriate receptor protein.
Behavioral and emotional influences of smell.
Transmission of Olfactory Signals:
From olfactory cells to glomeruli in the olfactory bulb, involving lateral inhibition.
Primitive, less old, and new olfactory systems with different path
Tom Selleck Health: A Comprehensive Look at the Iconic Actor’s Wellness Journey

Tom Selleck, an enduring figure in Hollywood. has captivated audiences for decades with his rugged charm, iconic moustache. and memorable roles in television and film. From his breakout role as Thomas Magnum in Magnum P.I. to his current portrayal of Frank Reagan in Blue Bloods. Selleck's career has spanned over 50 years. But beyond his professional achievements. fans have often been curious about Tom Selleck Health. especially as he has aged in the public eye.
Follow us on: Pinterest
Introduction
Many have been interested in Tom Selleck health. not only because of his enduring presence on screen but also because of the challenges. and lifestyle choices he has faced and made over the years. This article delves into the various aspects of Tom Selleck health. exploring his fitness regimen, diet, mental health. and the challenges he has encountered as he ages. We'll look at how he maintains his well-being. the health issues he has faced, and his approach to ageing .
Early Life and Career
Childhood and Athletic Beginnings
Tom Selleck was born on January 29, 1945, in Detroit, Michigan, and grew up in Sherman Oaks, California. From an early age, he was involved in sports, particularly basketball. which played a significant role in his physical development. His athletic pursuits continued into college. where he attended the University of Southern California (USC) on a basketball scholarship. This early involvement in sports laid a strong foundation for his physical health and disciplined lifestyle.
Transition to Acting
Selleck's transition from an athlete to an actor came with its physical demands. His first significant role in "Magnum P.I." required him to perform various stunts and maintain a fit appearance. This role, which he played from 1980 to 1988. necessitated a rigorous fitness routine to meet the show's demands. setting the stage for his long-term commitment to health and wellness.
Fitness Regimen
Workout Routine
Tom Selleck health and fitness regimen has evolved. adapting to his changing roles and age. During his "Magnum, P.I." days. Selleck's workouts were intense and focused on building and maintaining muscle mass. His routine included weightlifting, cardiovascular exercises. and specific training for the stunts he performed on the show.
Selleck adjusted his fitness routine as he aged to suit his body's needs. Today, his workouts focus on maintaining flexibility, strength, and cardiovascular health. He incorporates low-impact exercises such as swimming, walking, and light weightlifting. This balanced approach helps him stay fit without putting undue strain on his joints and muscles.
Importance of Flexibility and Mobility
In recent years, Selleck has emphasized the importance of flexibility and mobility in his fitness regimen. Understanding the natural decline in muscle mass and joint flexibility with age. he includes stretching and yoga in his routine. These practices help prevent injuries, improve posture, and maintain mobilit
Ozempic: Preoperative Management of Patients on GLP-1 Receptor Agonists 

Preoperative Management of Patients on GLP-1 Receptor Agonists like Ozempic and Semiglutide
ASA GUIDELINE
NYSORA Guideline
2 Case Reports of Gastric Ultrasound
How STIs Influence the Development of Pelvic Inflammatory Disease.pptx

STIs may cause PID. For the both disease, herbal medicine Fuyan Pill can be a solution.
For Better Surat #ℂall #Girl Service ❤85270-49040❤ Surat #ℂall #Girls

For Better Surat #ℂall #Girl Service ❤85270-49040❤ Surat #ℂall #Girls
Charaka Samhita Sutra sthana Chapter 15 Upakalpaniyaadhyaya

Charaka Samhita Sutra sthana Chapter 15 Upakalpaniyaadhyaya
TEST BANK for Operations Management, 14th Edition by William J. Stevenson, Ve...

TEST BANK for Operations Management, 14th Edition by William J. Stevenson, Verified Chapters 1 - 19, Complete Newest Version.pdf
TEST BANK for Operations Management, 14th Edition by William J. Stevenson, Verified Chapters 1 - 19, Complete Newest Version.pdf
How to Give Better Lectures: Some Tips for Doctors

Talk given to Koronadal Internists Society, Marbel, South Cotabato as part of the Continuing Medical Education (CME) series.
Non-respiratory Functions of the Lungs.pdf

These simplified slides by Dr. Sidra Arshad present an overview of the non-respiratory functions of the respiratory tract.
Learning objectives:
1. Enlist the non-respiratory functions of the respiratory tract
2. Briefly explain how these functions are carried out
3. Discuss the significance of dead space
4. Differentiate between minute ventilation and alveolar ventilation
5. Describe the cough and sneeze reflexes
Study Resources:
1. Chapter 39, Guyton and Hall Textbook of Medical Physiology, 14th edition
2. Chapter 34, Ganong’s Review of Medical Physiology, 26th edition
3. Chapter 17, Human Physiology by Lauralee Sherwood, 9th edition
4. Non-respiratory functions of the lungs https://academic.oup.com/bjaed/article/13/3/98/278874
ANATOMY AND PHYSIOLOGY OF URINARY SYSTEM.pptx

Valuable Content of Human Anatomy and Physiology of Urinary system as per PCI Syllabus for Pharmacy and PharmD Students.
Evaluation of antidepressant activity of clitoris ternatea in animals

Evaluation of antidepressant activity of clitoris ternatea in animals
Report Back from SGO 2024: What’s the Latest in Cervical Cancer?

Are you curious about what’s new in cervical cancer research or unsure what the findings mean? Join Dr. Emily Ko, a gynecologic oncologist at Penn Medicine, to learn about the latest updates from the Society of Gynecologic Oncology (SGO) 2024 Annual Meeting on Women’s Cancer. Dr. Ko will discuss what the research presented at the conference means for you and answer your questions about the new developments.
263778731218 Abortion Clinic /Pills In Harare ,

263778731218 Abortion Clinic /Pills In Harare ,ABORTION WOMEN’S CLINIC +27730423979 IN women clinic we believe that every woman should be able to make choices in her pregnancy. Our job is to provide compassionate care, safety,affordable and confidential services. That’s why we have won the trust from all generations of women all over the world. we use non surgical method(Abortion pills) to terminate…Dr.LISA +27730423979women Clinic is committed to providing the highest quality of obstetrical and gynecological care to women of all ages. Our dedicated staff aim to treat each patient and her health concerns with compassion and respect.Our dedicated group ABORTION WOMEN’S CLINIC +27730423979 IN women clinic we believe that every woman should be able to make choices in her pregnancy. Our job is to provide compassionate care, safety,affordable and confidential services. That’s why we have won the trust from all generations of women all over the world. we use non surgical method(Abortion pills) to terminate…Dr.LISA +27730423979women Clinic is committed to providing the highest quality of obstetrical and gynecological care to women of all ages. Our dedicated staff aim to treat each patient and her health concerns with compassion and respect.Our dedicated group of receptionists, nurses, and physicians have worked together as a teamof receptionists, nurses, and physicians have worked together as a team wwww.lisywomensclinic.co.za/
Recently uploaded (20)
Ocular injury ppt Upendra pal optometrist upums saifai etawah

Ocular injury ppt Upendra pal optometrist upums saifai etawah
NVBDCP.pptx Nation vector borne disease control program

NVBDCP.pptx Nation vector borne disease control program
Maxilla, Mandible & Hyoid Bone & Clinical Correlations by Dr. RIG.pptx

Maxilla, Mandible & Hyoid Bone & Clinical Correlations by Dr. RIG.pptx
Tom Selleck Health: A Comprehensive Look at the Iconic Actor’s Wellness Journey

Tom Selleck Health: A Comprehensive Look at the Iconic Actor’s Wellness Journey
Triangles of Neck and Clinical Correlation by Dr. RIG.pptx

Triangles of Neck and Clinical Correlation by Dr. RIG.pptx
Ozempic: Preoperative Management of Patients on GLP-1 Receptor Agonists 

Ozempic: Preoperative Management of Patients on GLP-1 Receptor Agonists
How STIs Influence the Development of Pelvic Inflammatory Disease.pptx

How STIs Influence the Development of Pelvic Inflammatory Disease.pptx
For Better Surat #ℂall #Girl Service ❤85270-49040❤ Surat #ℂall #Girls

For Better Surat #ℂall #Girl Service ❤85270-49040❤ Surat #ℂall #Girls
Charaka Samhita Sutra sthana Chapter 15 Upakalpaniyaadhyaya

Charaka Samhita Sutra sthana Chapter 15 Upakalpaniyaadhyaya
TEST BANK for Operations Management, 14th Edition by William J. Stevenson, Ve...

TEST BANK for Operations Management, 14th Edition by William J. Stevenson, Ve...
How to Give Better Lectures: Some Tips for Doctors

How to Give Better Lectures: Some Tips for Doctors
Evaluation of antidepressant activity of clitoris ternatea in animals

Evaluation of antidepressant activity of clitoris ternatea in animals
Report Back from SGO 2024: What’s the Latest in Cervical Cancer?

Report Back from SGO 2024: What’s the Latest in Cervical Cancer?
Viruses and vaccine production
- 1. Viral vaccine and production
- 2. CONTENT 1. What is a vaccine ? 2. Classical vaccines & engineered vaccines 3. Replicating versus inactivated vaccines 4. Purpose of vaccination 5. Crucial aspect defining the process choice 6. Host cell characteristics & growth requirements 7. Advantages & disadvantages of insect cells 8. Virus/antigen stability 9. Vaccine production 10. Vaccine production in bioreactors 11. Process intensification in bioreactors 12. Manufacturing basics
- 3. 1. WHAT IS A VACCINE ? • Biological preparation providing Active Acquired Immunity • Goal of vaccination: to stimulate the body to develop a specialized, protective, immune response in the absence of disease. • Immune responses target viral proteins which can be delivered as : i. Weakened (attenuated) viruses that replicate in the host without causing disease. ii. Inactivated (killed) virions. iii. Purified protein products. iv. Nucleic acids to direct synthesis of desired proteins.
- 4. 2. CLASSICAL VACCINES & ENGINEERED VACCINES CLASSICAL VACCINES ENGINEERED VACCINES 1. Attenuated viruses usually obtained by repeated passage in animals or cultured cells. 1. provide a safe source of antigen, representative of a particular pathogen. 2. Unpredictable results and inadequately attenuated viruses. 2.1 Virulent viruses weakened by deletion/modifications of specific genes 2.2 Genes for capsid or envelope proteins can be transferred to a safer virus. 2.3 genes of virulent virus moved into bacteria, yeast, or cultured cells to produce large quantities of a particular viral protein. 2.4 administer DNA (cloned genes) directly into the recipient. 3. possibility of virus reverting to virulence. 3. use of adjuvants
- 5. 3. REPLICATING VERSUS INACTIVATED VACCINES LIVING VACCINE INACTIVATED VACCINE 1. Fewer, smaller doses and adjuvant unnecessary 1. Do not replicate in recipient 2. Cheap to produce 2. Lower cost to develop 3. Longer protection and less chance of hypersensitivity. 3. Unlikely to cause disease through residual virulence 4. Stable on storage
- 6. 4.Purpose of vaccination • Stimulate the production of neutralizing antibodies for the purpose of blocking infection • Neutralizing antibodies might - i. prevent a virus from binding to a receptor ii. prevent membrane fusion iii. prevent un-coating of the viral genome.
- 7. 5.Crucial aspect defining the process choice 5.1 VACCINE DEMAND: i. Spread of a virus and its mutation rate. ii. Time period Ex: seasonal influenza a time period as short as 5–6 months. iii. Polio or measles require a more or less constant supply of the same vaccine strain for worldwide application. iv. Vaccines against dengue or yellow fever are only needed in certain regions of the world.
- 8. 5.2 VACCINE TYPE i. Live attenuated viruses, inactivated viruses, virus subunits, viral vectors or recombinant virus-like particles/proteins ii. Viruses with a high mutation rate are unsuitable candidates for live attenuated vaccine type as reversion may occur during vaccine production. iii. Attenuated virus strains in cell culture gives lower process yield iv. Wild-type live viruses require biosafety level 3 v. Recombinant vaccines may require higher antigen concentrations per dose
- 11. 5.3 VIRUS/ANTIGEN REQUIREMENTS FOR GROWTH/EXPRESSION i. Chemically defined protein-free media ii. Hepatitis A (strain HM175) propagated in MRC-5 human diploid cells iii. Recombinant hepatitis B surface antigen (HBsAg) produced in yeast cells grown in a complex medium of extract of yeast, soy peptone, dextrose, amino acids, and mineral salts iv. Measles virus propagated in chick embryo cell culture
- 12. 6. HOST CELL CHARACTERISTICS & GROWTH REQUIREMENTS : i. Right cell line & optimal process parameter conditions e.g., temperature, pH value, dissolved oxygen concentration, medium composition, etc. ii. Human/higher animal cell substrates for whole virus replication. iii. Insect cell substrates for recombinant antigen and virus-like particle (VLP) production. iv. Continuous cell lines preferred over primary cells : Vero, MDCK, MRC-5, WI-38, HEK293, PER.C6, AGE1.CR ,EB66 v. Media development- serum free
- 13. 7. ADVANTAGES & DISADVANTAGES OF INSECT CELLS : i. Suspension cultures using serum-free media ii. Insect cell-baculovirus expression system iii. Difficultly to express membrane proteins , secreted glycoproteins at appropriate levels or complex glycan structures 8. VIRUS/ANTIGEN STABILITY : i. Hollow-fiber-based perfusion systems enables selective separation and further processing of the product-containing medium. ii. To avoid virus/antigen degradation in upstream processing, which is mainly caused by the release of cellular proteases after cell lysis low thermo-stabilities.
- 14. 9. VACCINE PRODUCTION Roller bottles i. A practical and low-cost option for cell culture at laboratory scale and for large-scale manufacturing of products ii. Automated handling of up to 1000 RBs per batch (IDT Biologika)
- 16. 10. VACCINE PRODUCTION IN BIOREACTORS DISCONTINUOUS BATCH CULTIVATION MODE i. Low instrumental and operational intervention ii. Good virus yield coefficients and high nutrient consumption iii. Fed-batch or perfusion systems aims on higher cell concentrations & increased volumetric virus yields
- 17. 11. PROCESS INTENSIFICATION IN BIOREACTORS i. Microcarriers ii. Fixed-bed systems or packed-bed systems iii. Shake flasks , wave bioreactors or STRs iv. Fed-batch mode v. Perfusion systems (external hollow-fiber) vi. Continuous bioreactor vii. Use of ‘Single-use bioreactors’ in vaccine production
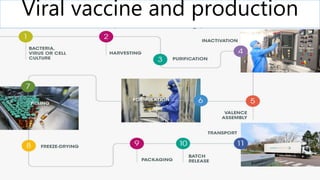
- 18. 12. MANUFACTURING BASICS GENERATION of the pathogen recombinant protein derived from the pathogen RELEASE the antigen from the substrate and isolate it from the bulk of the environment PURIFICATION of the antigen unit operations of column chromatography & ultrafiltration inactivation of isolated virus. FORMULATION of the vaccine include an adjuvant QUALITY CONTROL (QC) testing safety, potency, purity, sterility, and other assays specific to the product STORAGE addition of stabilizers or lyophilization , very low temperatures