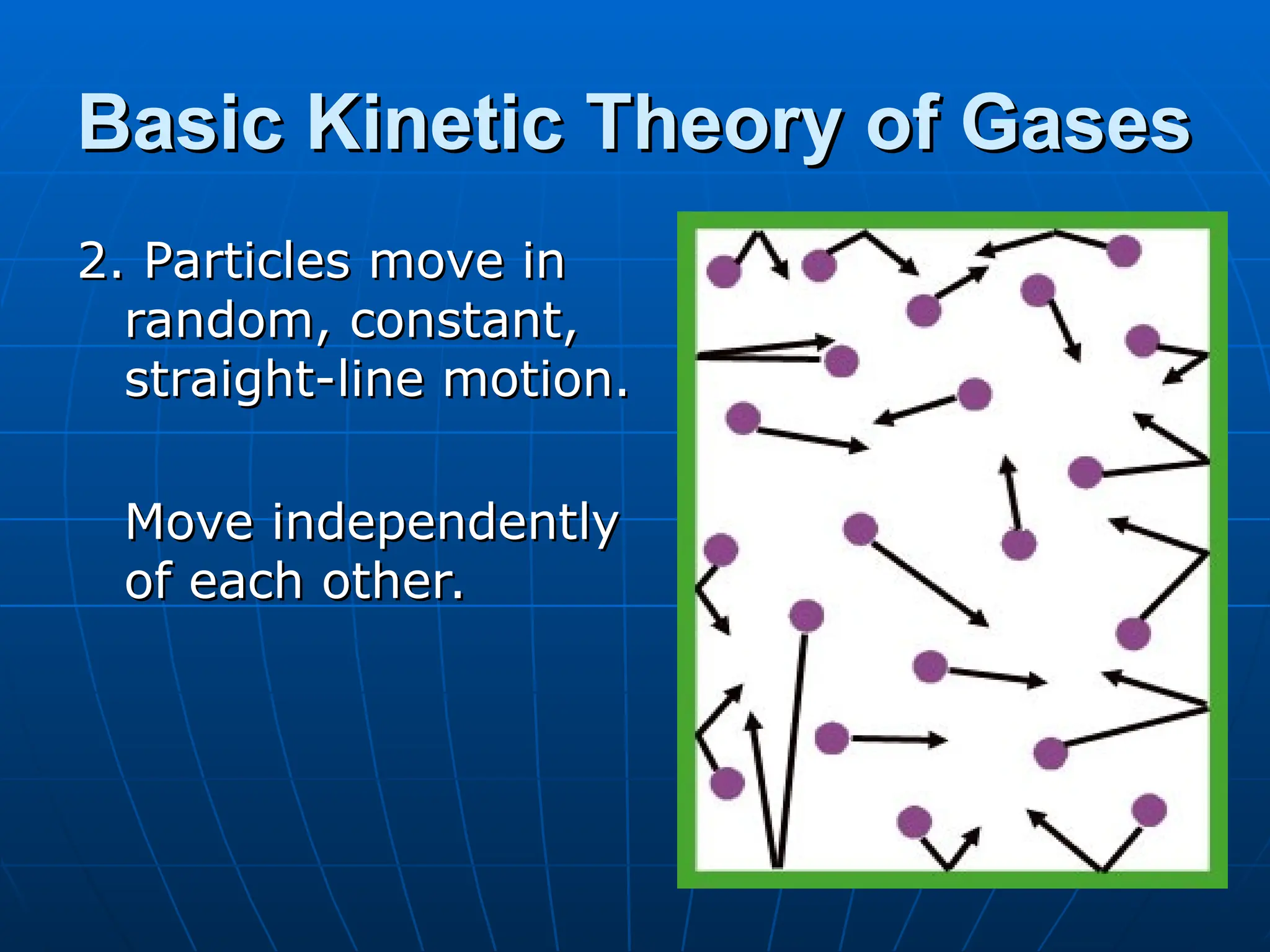
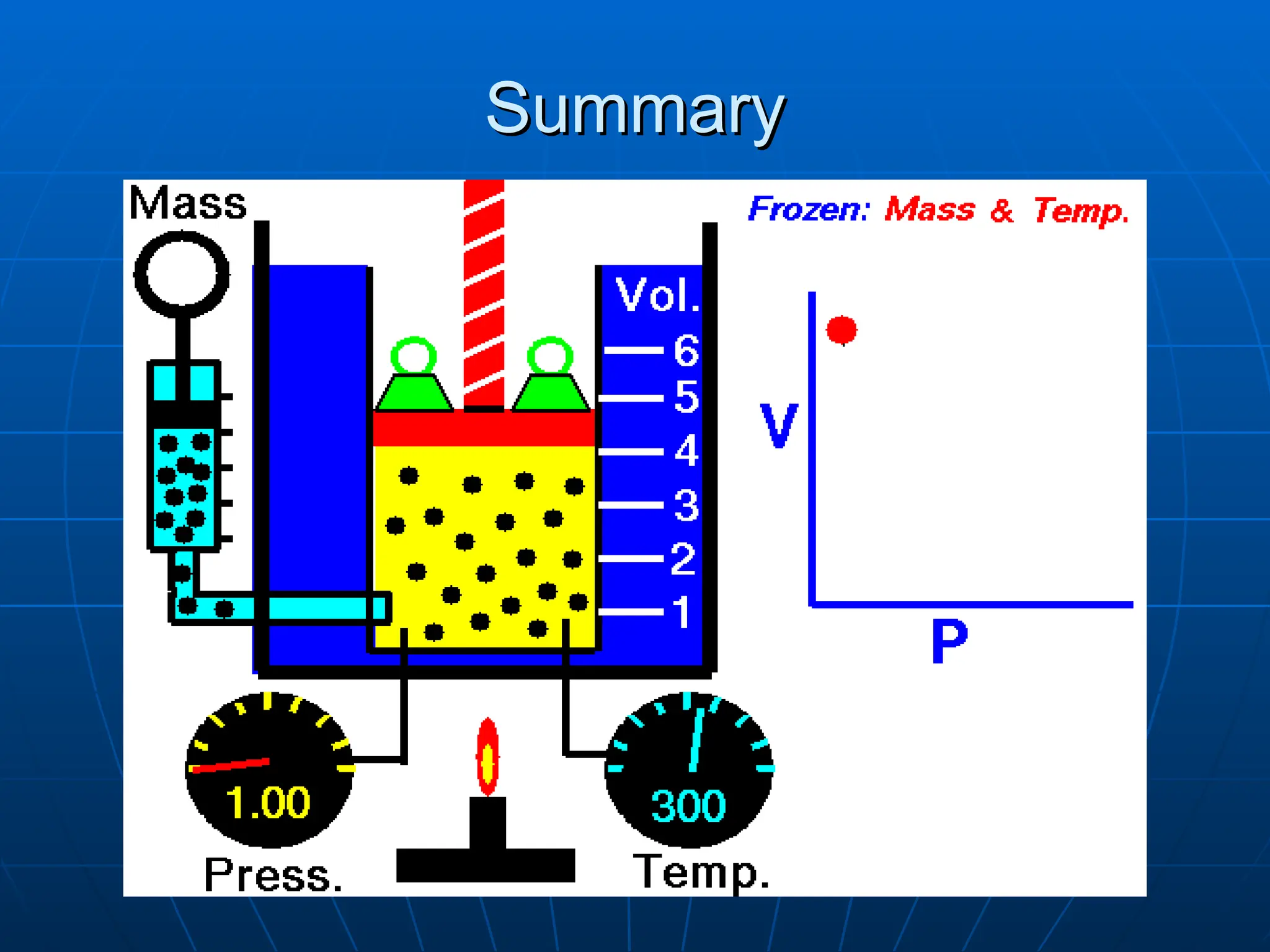
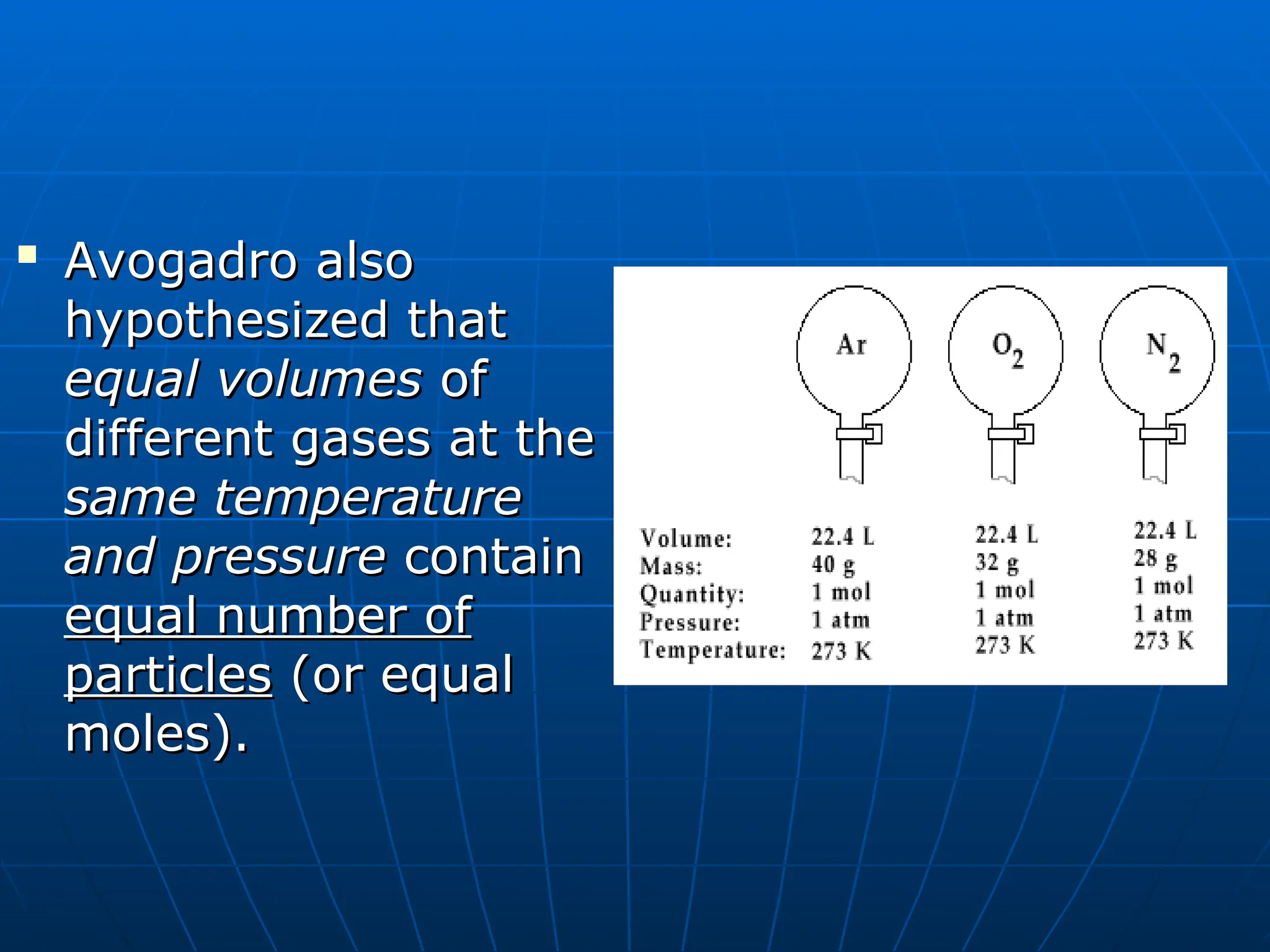
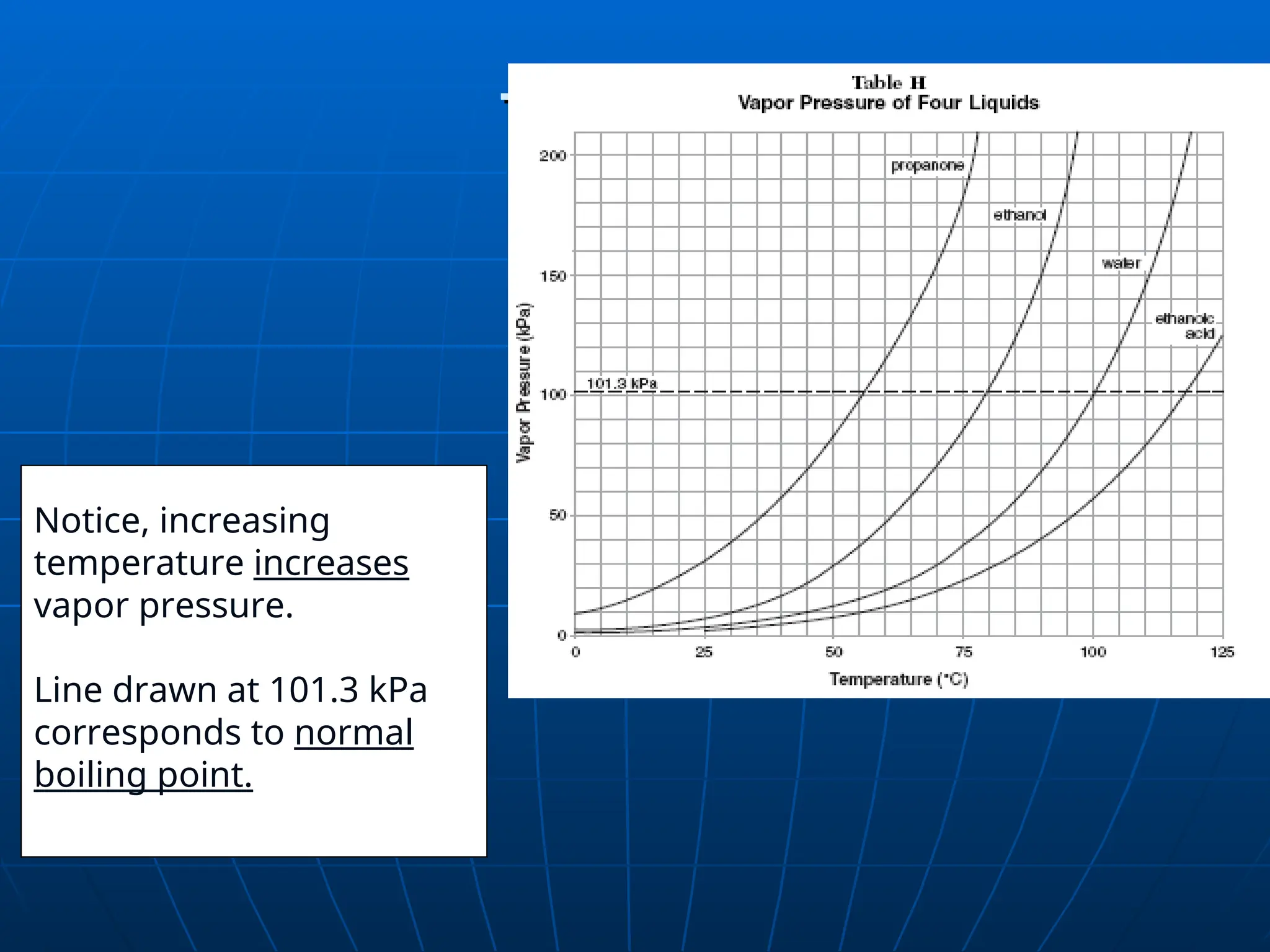
The document discusses the properties and behavior of gases, including the kinetic molecular theory, gas laws, and differences between ideal and real gases. Key concepts include the relationships between pressure, volume, and temperature, as outlined in Boyle's, Charles', and Gay-Lussac's laws. Additionally, it addresses vapor pressure, evaporation, and boiling points, emphasizing the influence of intermolecular forces on boiling temperatures.