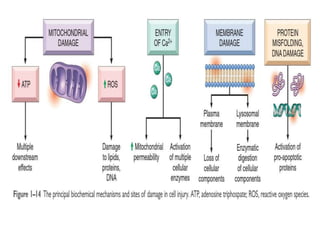
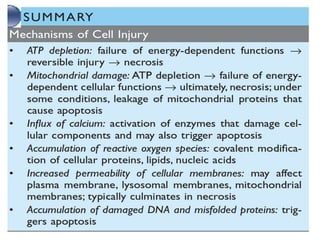
This document summarizes the key mechanisms of cellular injury:
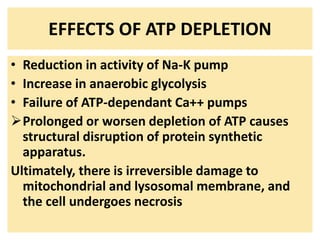
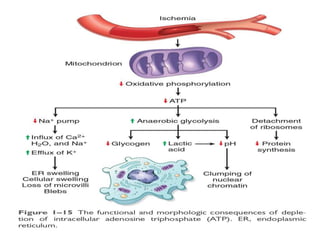
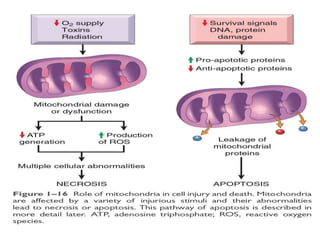
1. Depletion of ATP disrupts cellular processes and leads to necrosis as mitochondria and lysosomes are damaged. ATP depletion occurs due to reduced oxygen/nutrients, mitochondrial damage, or toxins.
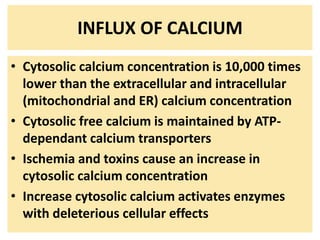
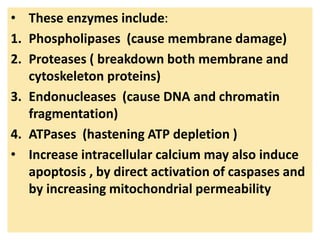
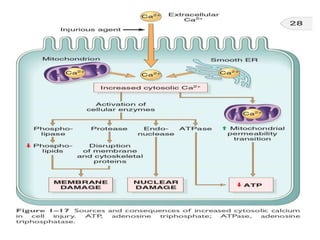
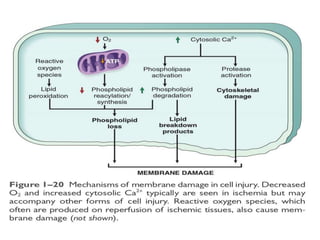
2. Influx of calcium into cells activates destructive enzymes and can induce apoptosis. Increased cytosolic calcium occurs when ATP-dependent calcium pumps fail during ischemia or toxicity.
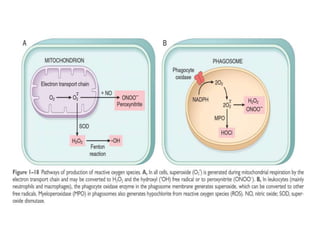
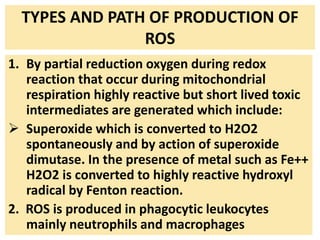
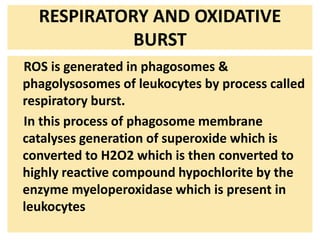
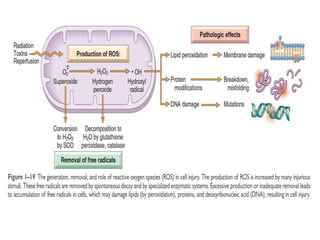
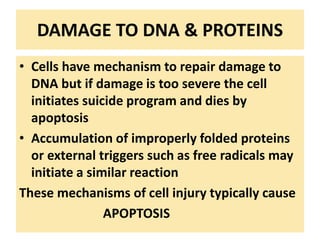
3. Accumulation of reactive oxygen species damages lipids, proteins, and DNA, causing cell injury. ROS are generated during oxidative phosphorylation and inflammation and cause oxidative stress.