Physiology is the study of normal cell, tissue, organ, and system function. The document outlines several key topics in physiology including:
1) Cell physiology focuses on cell function and interactions, while molecular physiology examines subcellular processes. Other specialties study specific organs or systems.
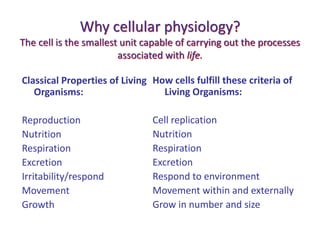
2) Cells are the basic functional units of the body and fulfill the criteria of living organisms through processes like nutrition, respiration, and growth.
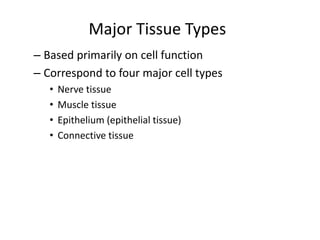
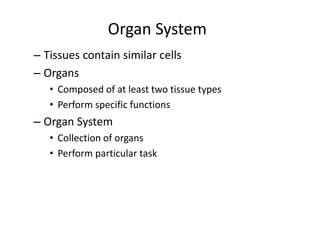
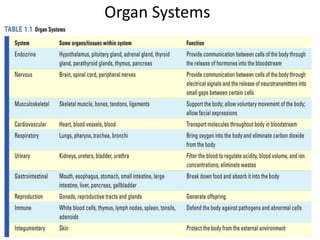
3) The human body contains over 200 cell types organized into tissues, organs, and organ systems like the nervous and circulatory systems.
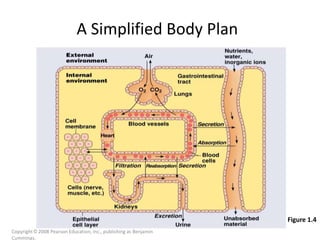
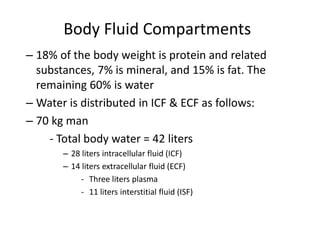
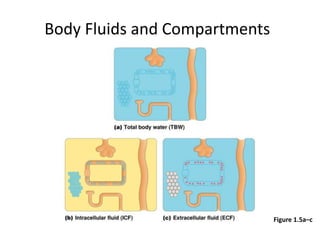
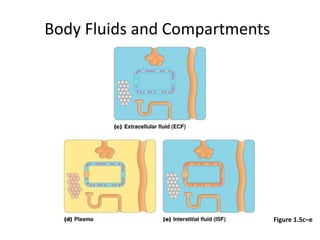
4) Body fluids are distributed into intracellular and extracellular compartments, which are maintained within tight concentration ranges through diffusion and buffering processes.






![pH AND BUFFERING
• The maintenance of a stable hydrogen ion
concentration ([H+]) in body fluids is essential to life
• Is the negative logarithm of the [H+]
• Substance donating H+ in solutions are acids
• Body pH is stabilized by the buffering capacity of
the body fluids
• isohydric principle
• Henderson Hasselbach equation:
pH = pKa + log [A–]/[HA]](https://image.slidesharecdn.com/introductiontophysiology-170517175512/85/Introduction-to-physiology-16-320.jpg)


![• NERNEST EQUATION: What membrane
potential would exist at the true equilibrium
for a particular ion? & What is the voltage that
would balance diffusion gradients with the
force that would prevent net ion movement?
• This theoretical equilibrium potential can be
calculated (for a particular ion)
RT [Na+]out
[Na+]in
ENa =
zF
ln
___ ___
For K+ around -90mV
For Na+ around +60mV](https://image.slidesharecdn.com/introductiontophysiology-170517175512/85/Introduction-to-physiology-20-320.jpg)