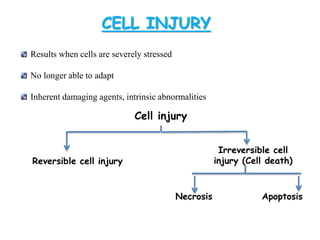
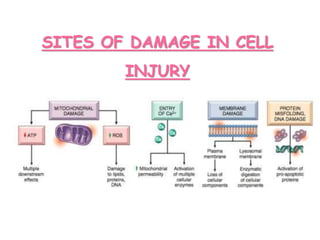
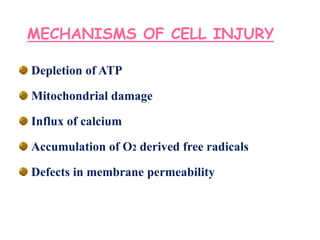
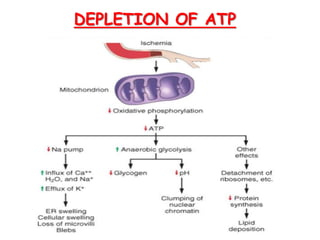
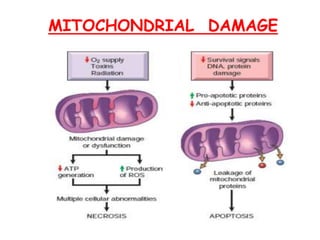
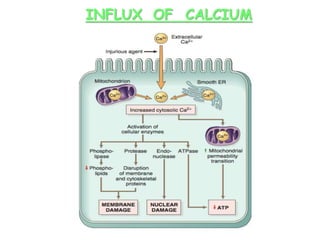
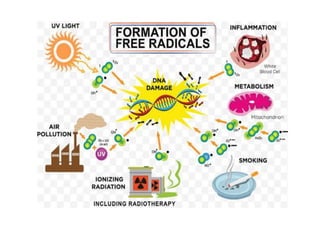
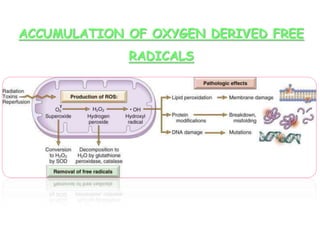
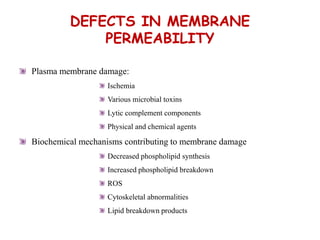
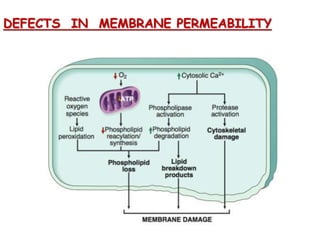
The document discusses cell injury and its mechanisms, distinguishing between reversible and irreversible injuries, including necrosis and apoptosis. It emphasizes the role of ATP depletion, mitochondrial damage, calcium influx, and the accumulation of reactive oxygen species in causing cell injury and death. The text outlines how oxidative stress and membrane permeability defects contribute to cellular damage and the physiological and pathological contexts in which these processes occur.