Ved Prakash Sharma is a final year B.Tech student specializing in mechanical engineering, who has presented research on erosion-corrosion in power plants. The document discusses causes, prevention techniques, and coating methods to mitigate these issues, highlighting the importance of surface treatment and thermal spray techniques. Conclusions emphasize that a composite coating system is optimal for protecting components against high-temperature erosion and corrosion.


![Introduction
Corrosion is the degradation of material due to
chemical interaction with their environment.
According to Khanna (2005), oxidation - is the formation
of the oxide scale. If the scale spalls frequently - metal is
consumed continuously - material ultimately fails [1]
.
Erosion is the degradation of material due to
mechanical interaction with the environment.
He et al.,(2001), reported that both mechanical properties
and oxidation resistance must be considered for its use in
high temperature applications [2]
.](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-3-320.jpg)
![ Chatterjee et al., (2001), - Establishment and maintenance of an
impervious, stable, inert, adherent, protective layer on the substrate
during the service period [3]
.
Heath et al., (1997), - Material damage can be controlled by including
proper alloy selection, optimum design of components, injecting chemical
additives, shielding of substrates and protective coatings [4]
.
Eliaz et al., (2002), - Hot corrosion of gas turbine components could be
controlled by employing proper selection of structural alloys, application of
coatings [5]
.
Priyantha et al., (2003), - An estimated 40% of total US steel
production goes to replacement of corroded parts and products [6]
.
Cheruvu et al., (2006), – replacement cost of the hot section components
can exceed 35% of the cost of a new plant [7]
.
Otsuka (2002), - Condensation/accumulation of low melting-point salts
from flue-gas - root cause for the severe wastage of tube materials [8]
.](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-4-320.jpg)
![Boiler elements highly vulnerable to erosion–corrosion
wear (Szymański et al., 2015)[9]](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-5-320.jpg)
![Typical examples of erosive wear in fluidized boiler: a) the area over the
ceramic lining, b) transition zone in thermally sprayed coatings, c) damage
caused by erosion of the wall, and d) damaged superheater tube.[9]](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-6-320.jpg)

![Thermal spray
technique:-
Melted particles
Voids, Porosity
Un-Melted Particles
Schematic diagram of thermal spray process, Sidhu et al., (2007) [10]](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-8-320.jpg)
![Coating parameters of thermal spray process (Kuroda et al.,
2008) [11]
.](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-9-320.jpg)


![Type of Salt Deposit found on Boiler for
different fuels.
Type of fuel Typical salt deposits References
Waste ZnCl2, PbCl2, KCl, NaCl Smith et al., (2001) [12]
,
Spiegel et al., (2000) [13]
Straw KCl, K2SO4 Montgomery et al., (2000) [14]
Wood KCl, K2SO4, NaCl, Na2SO4 Henderson et al., (2000) [15]
Residual oil Na2SO4, V2O5 Luthra et al., (1982) [16]
Coal Na2SO4, K2SO4,
(Na,K)2Fe(SO4)3
Reichel et al., (1988) [17]
Viswanathan et al., (2002) [18]](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-12-320.jpg)
![Melting Points of Salt Deposits on Boiler
Tube (George et al.,2007) [19]](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-13-320.jpg)
![A CASE STUDY ON BOILER TUBE FAILURE by Prakash et al., (2001) [20]
S. NO. Type of Failure No. of Failures %age
out of 89
1 Erosion due to ash and hot 50 56.18
corrosion including overheating
due to corrosion
2 Erosion due to pulverized fuel 12 13.48
from coal nozzles
3 Welding joint cracks 10 11.24
4 Overheating due to choking 8 8.99
5 Leakage from water wall, header 5 5.62
drains due to expansion and
contraction
6 Miscellaneous 4 4.49](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-14-320.jpg)





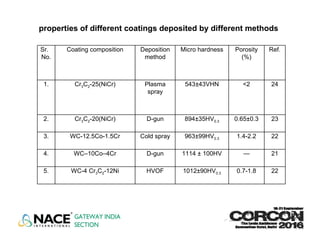


![REFERENCES
[1] Khanna, A. S., (2005), Ch. 6: “Handbook of environmental degradation of Materials”, Pub., William Andrew, 13 Eaton Avenue,
New York.
[2] He, J. L., Chen K.C., Chen C.C., Leyland A., and Matthews A., (2001), “Cyclic oxidation resistance of Ni-Al alloy coatings
deposited on steel by a cathodic arc plasma process”, Surf. Coat. Technol., Vol. 135, pp. 158-165.
[3] Chatterjee, U. K, Bose S. K., and Roy S. K., (2001), “Environmental Degradation of Metals”, Pub., Marcel Dekkar, Inc. 270
Madison Avenue, New York.
[4] Heath, G. R., Heimgartner, P., Irons, G., Miller, R. and Gustafsson, S.,(1997), “An Assessment of Thermal Spray Coating
Technologies for High Temperature Corrosion Protection,” Mater. Sci. Forum, Vol. 251-54, pp. 809-816.
[5] Eliaz, N., Shemesh G., and Latanision R. M., (2002), “Hot corrosion in gas turbine components”, Engineering Failure Analysis,
Vol. 9, pp. 31-43.
[6] Priyantha, N., Jayaweera, P., Sanjurjo, A., Lau, K., Lu, F. and Krist, K., (2003), “Corrosion-Resistant Metallic
Coatings for Applications in Highly Aggressive Environments,” Surf. Coat. Technol., Vol. 163-164, pp. 31-36.
[7] Cheruvu N.S., Chan K.S., and Viswanathan R., (2006), “Evaluation, degradation and life assessment of coatings for
land based combustion turbines,” Energy Mater., Vol. 1, No. 1, pp. 33-47.
[8] Otsuka, N, (2002), “Effects of Fuel Impurities on the Fireside Corrosion of Boiler Tubes in Advanced Power
Generating Systems-A Thermodynamic Calculation of Deposit Chemistry,” Corros. Sci., Vol 44, pp. 265-283.
[9] Szymański Krzysztof, Hernas Adam, Moskal Grzegorz and Myalska Hanna, Thermally sprayed coatings resistant to
erosion and corrosion for power plant boilers - A review, Surf. Coat. Technol., Vol. 268, (2015), pp. 153-164
[10] Sidhu T S, Malik A, Prakash S, Agrawal R D, “ Oxidation and Hot Corrosion Resistance of HVOF WC-NiCrFeSiB
Coating on Ni- and Fe-based Superalloys at 800 °C,” Journal Thermal Spray Technology, vol. 16(5-6), pp. 844-849,
2007.
[11] Kuroda, S., Kawakita, J., Watanabe, M. and Katanoda, H. (2008), “Topical Review Warm spraying- A Novel
Coating Process Based on High-Velocity Impact of Solid Particles,” Sci. Technol. Adv. Mater., Vol. 9, pp. 1-17.
[12] Smith R.J.,Farr N.C.,Baker B.A.,the corrosion resistance of high nickel based alloys in relation to waste
incineratore applications, Proceedings of the Eurrcorr 2001, 30 sep-4 oct 2001, Rivadel garda, lake garda , Italy (2001)](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-23-320.jpg)
![[13] Spiegel M., Zahs A., Grabke H.J., Fundamental aspects of chlorine induced corrosion in power plants, Materials at
high temperature, 2 (2000) p 153-159.
[14]Montgomery M., Karlsson A., Larsen O.H.,In situ corrosion experiments at various strawfired power plants in
Denmark, Proceedings of the Eurocorr 2000, 10th 14th september 2000, Queen Mary westfield college university of
london UK (2000)
[15]Henderson P.J., Ljung P., Kallner P., Tollin J., Fireside corrosion of superheater materials in a wood fired
circulating fluidised bed boiler, Proceedings of the Eurocorr 2000, 10th-14th september 2000, Queen Marry &
Westfieldcollege university of London UK (2000).
[16] Luthra, K.L., Spacil, H.S.,IMPURITY DEPOSITS IN GAS TURBINES FROM FUELS CONTAINING SODIUM AND
VANADIUM. Journal of the Electrochemical Society Volume 129, Issue 3, March 1982, Pages
[17] Reichal H.H,Fireside corrosion in german fossil fuel fired power plants, Appearance, mechanism and causes,
Werkstoffe and Korrosion, 39 (1988) p 54-63
[18] Viswanathan R.,Purget R., Rao U, Materials for ultra supercritical coal fired power plant boilers, Proceedings of the
7th liege conference on Materials for advanced power engineering, Energy, 2002.
[19] George Y. Lai, “ High Temperature corrosion and materials application” , ASM International, 2007.
[20] Prakash, S., Singh, S., Sidhu, B. S. and Madeshia, A., (2001), “Tube Failures in Coal Fired Boilers,” Proc. National
Seminar on Advances in Material and Processing, Nov., 9-10, I1TR, Roorkee, India, pp. 245-253.
[21] Robert J.K. Wood, “Tribology of thermal sprayed WC–Co coatings,” Int. Journal of Refractory Metals & Hard
Materials Vol. 28, pp. 82–94, 2010.
[22] Berger L.-M., Saaro S., Naumann T., Wiener M., Weihnacht V., Thiele S. and Suchánek J., “Microstructure and
properties of HVOF sprayed chromium alloyed WC–Co and WC– Ni coatings,” Surface & Coatings Technology, vol.
202, pp.4417–4421, 2008.
[23] Murthy J.K.N. and Venkataraman B., “Abrasive wear behavior of WC–CoCr and Cr3C2–20(NiCr) deposited by
HVOF and detonation spray processes,” Surface & Coatings Technology, vol. 200, pp. 2642– 2652, 2006.
[24] Factor M. and Roman I., “Microhardness as a Simple Means of Estimating Relative Wear Resistance of Carbide
Thermal Spray Coatings,” Part 1 Characterization of Cemented Carbide Coatings ASM International JTTEE5, vol. 11,
pp. 468-481, 2001.
[25] Kamal S, Jayaganthan R and Prakash S, “Evaluation of cyclic hot corrosion behavior of detonation gun sprayed
Cr3C2–25%NiCr coatings on nickel- and iron-based super alloys,” Surf. Coat. Technol., vol. 203, pp. 1004–1013, 2009.](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-24-320.jpg)
![[26] Guilemany J.M., Espallargas N., Suegama P.H. and Benedetti A.V., “Comparative study of Cr3C2–NiCr coatings
obtained by HVOF and hard chromium coatings,” Corrosion Science, vol. 48, pp. 2998–3013, 2006.
[27] Suarez M., Bellayer S., Traisnel M., Gonzalez W., Chicot D., Lesage J., Puchi Cabrera E.S. and Staia M.H.,
“Corrosion behavior of Cr3C2-NiCr vacuum plasma sprayed coatings,” Surface & Coatings Technology, vol. 202, pp.
4566–4571, 2008.
[28] Chatha S. S., Sidhu H. S. and Sidhu B. S., “The effects of post-treatment on the hot corrosion behaviour of the
HVOF-sprayed Cr3C2-NiCr coating,” Surface & Coatings Technology, vol. 206, pp. 4212-4224, 2012.
[29] Kaur M., Singh H. and Prakash S., “Role of detonation gun spray Cr3C2-NiCr coating in improving high temperature
corrosion resistance of SAE-213-T22 and SAE-347H steel in presence of Na2SO4-Fe2(SO4)3 salt deposits,” Surface
Engineering, pp. 1-12, 2009.
[30] Kaur M., Singh H. and Prakash S, “High-temperature corrosion studies of HVOF- sprayed Cr3C2-NiCr coatings on
SAE-347H boiler steel,” Journal of Thermal Spray Technology, Volume 18(4), pp. 619-632, December 2009.](https://image.slidesharecdn.com/885ppt30vedprakashsharmappt-170520101241/85/Corrosion-and-Erosion-25-320.jpg)