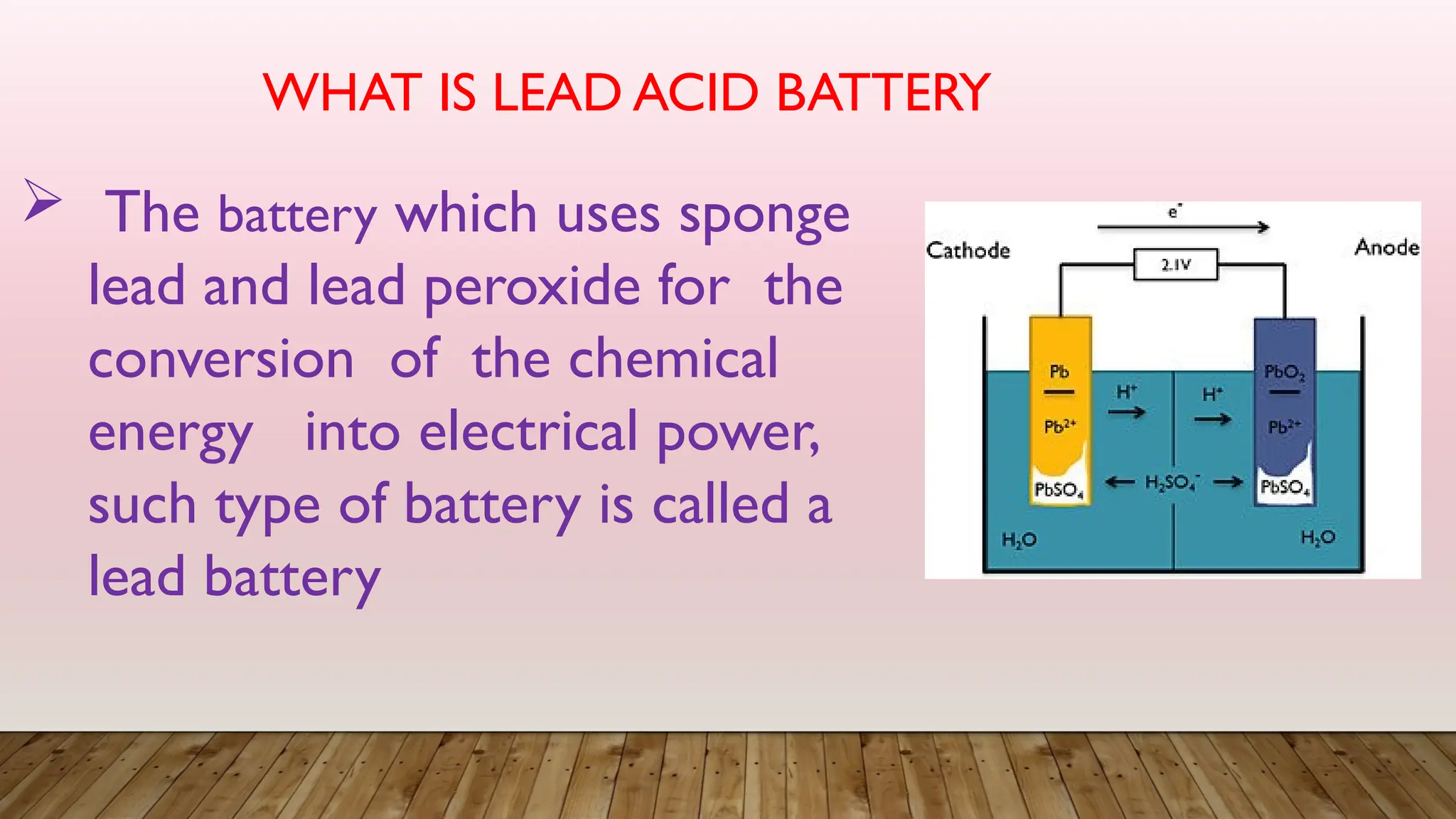
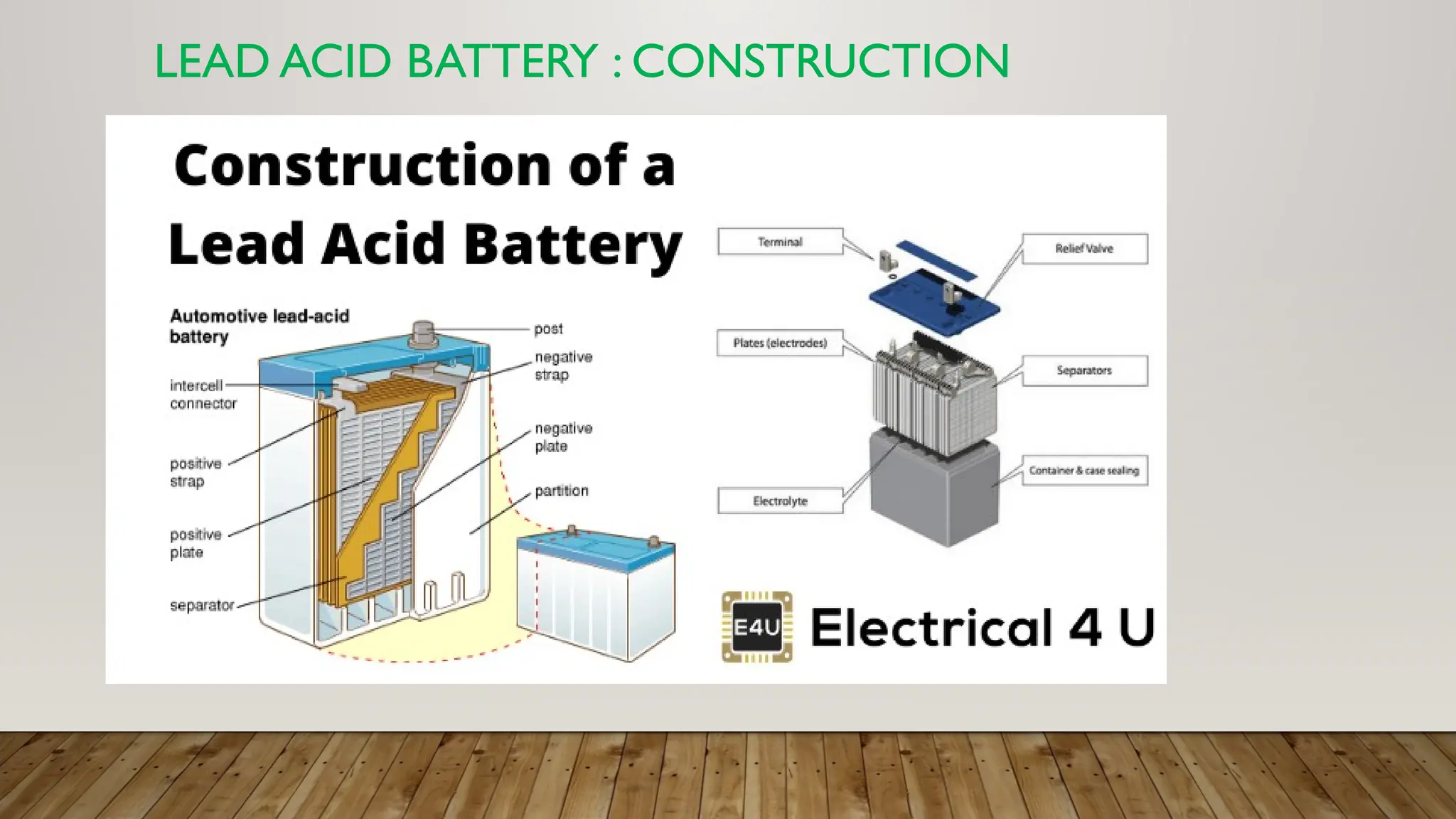
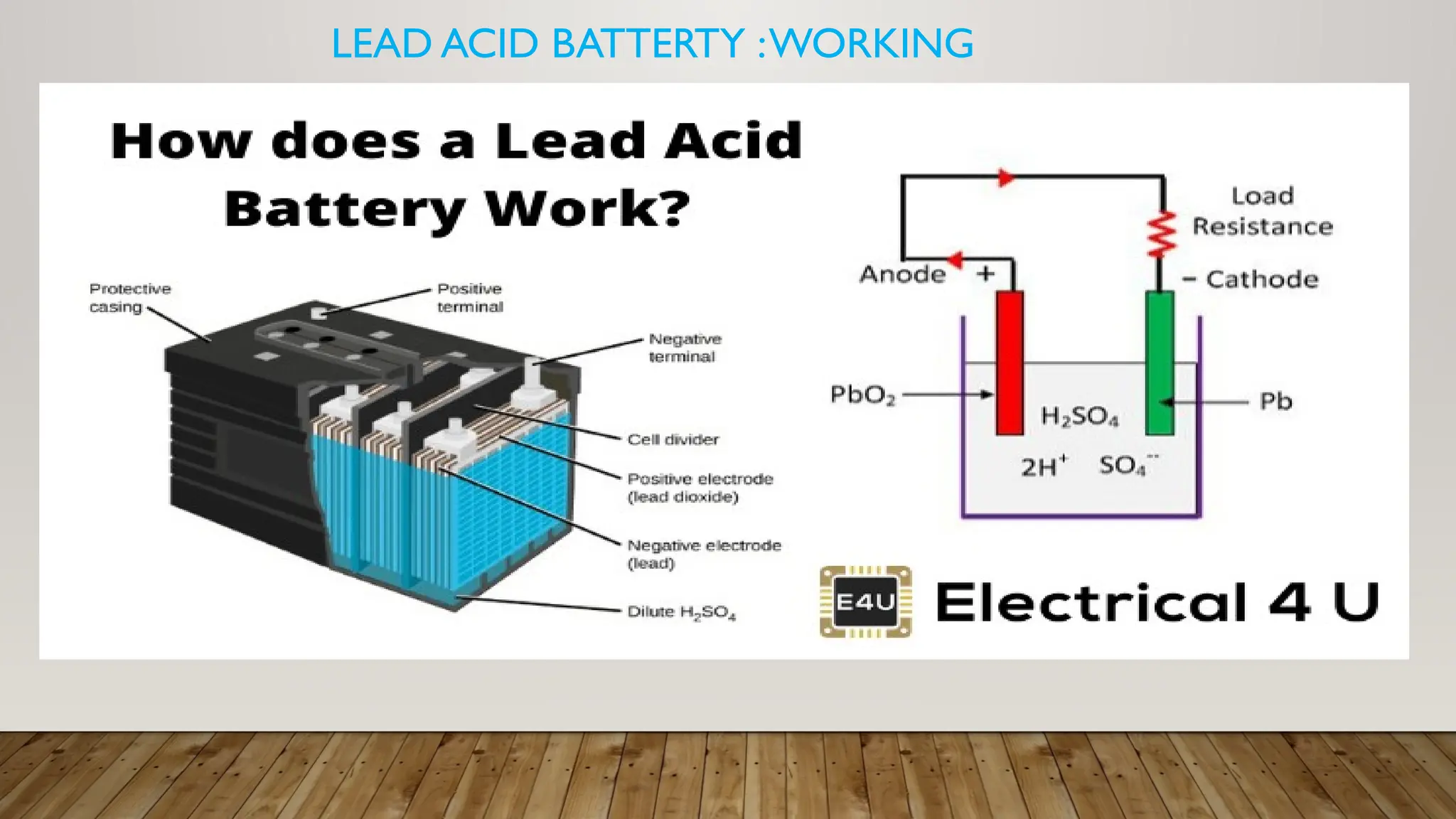
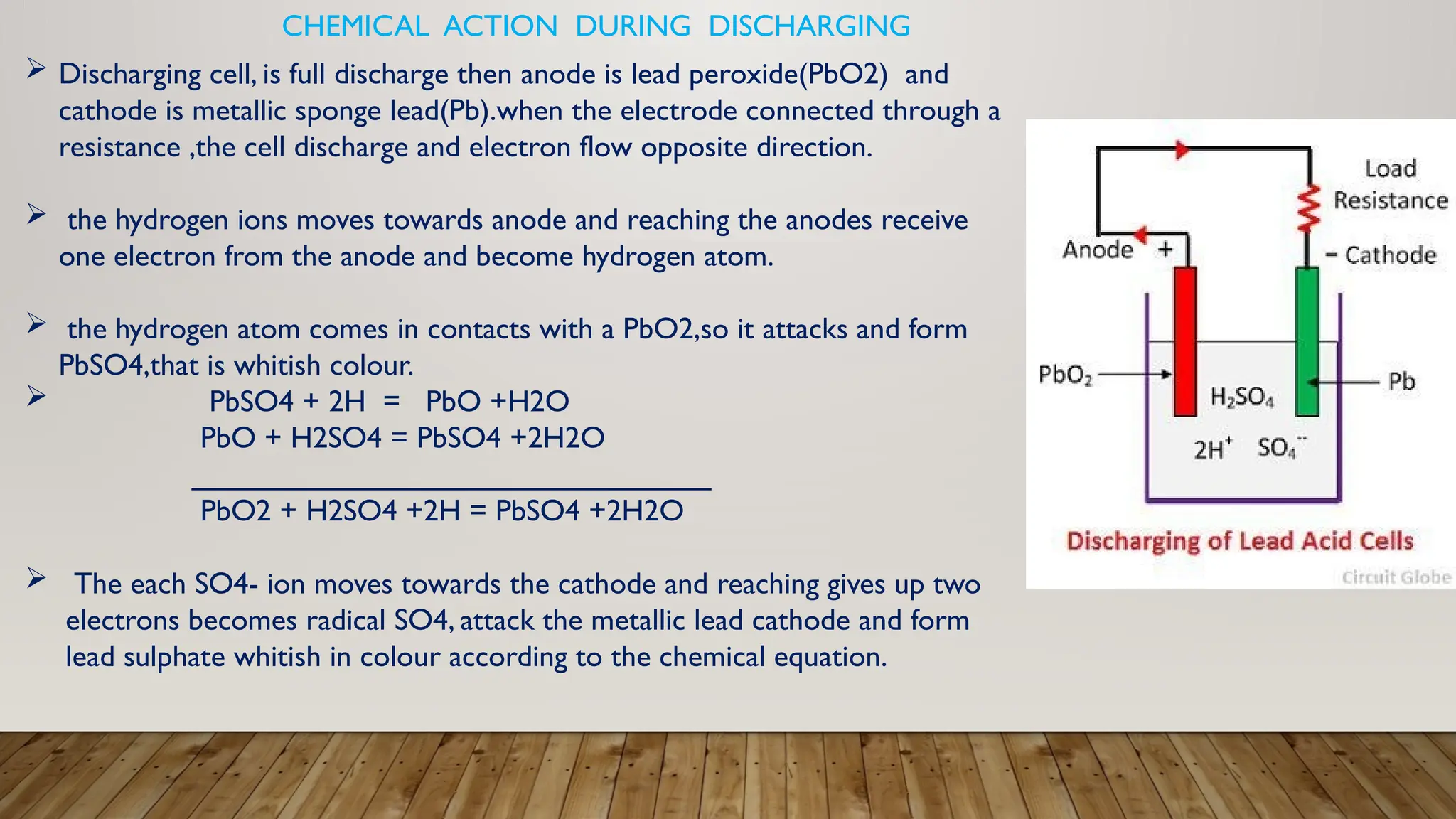
The document provides an overview of lead acid batteries, detailing their construction, working principle, active materials, charging and discharging processes, maintenance, applications, advantages, and disadvantages. It describes the chemical reactions involved during both charging and discharging, as well as practical maintenance tips and uses in various applications. Lead acid batteries are characterized by their ability to produce high currents, affordability, but also have limitations such as low specific energy and environmental concerns.