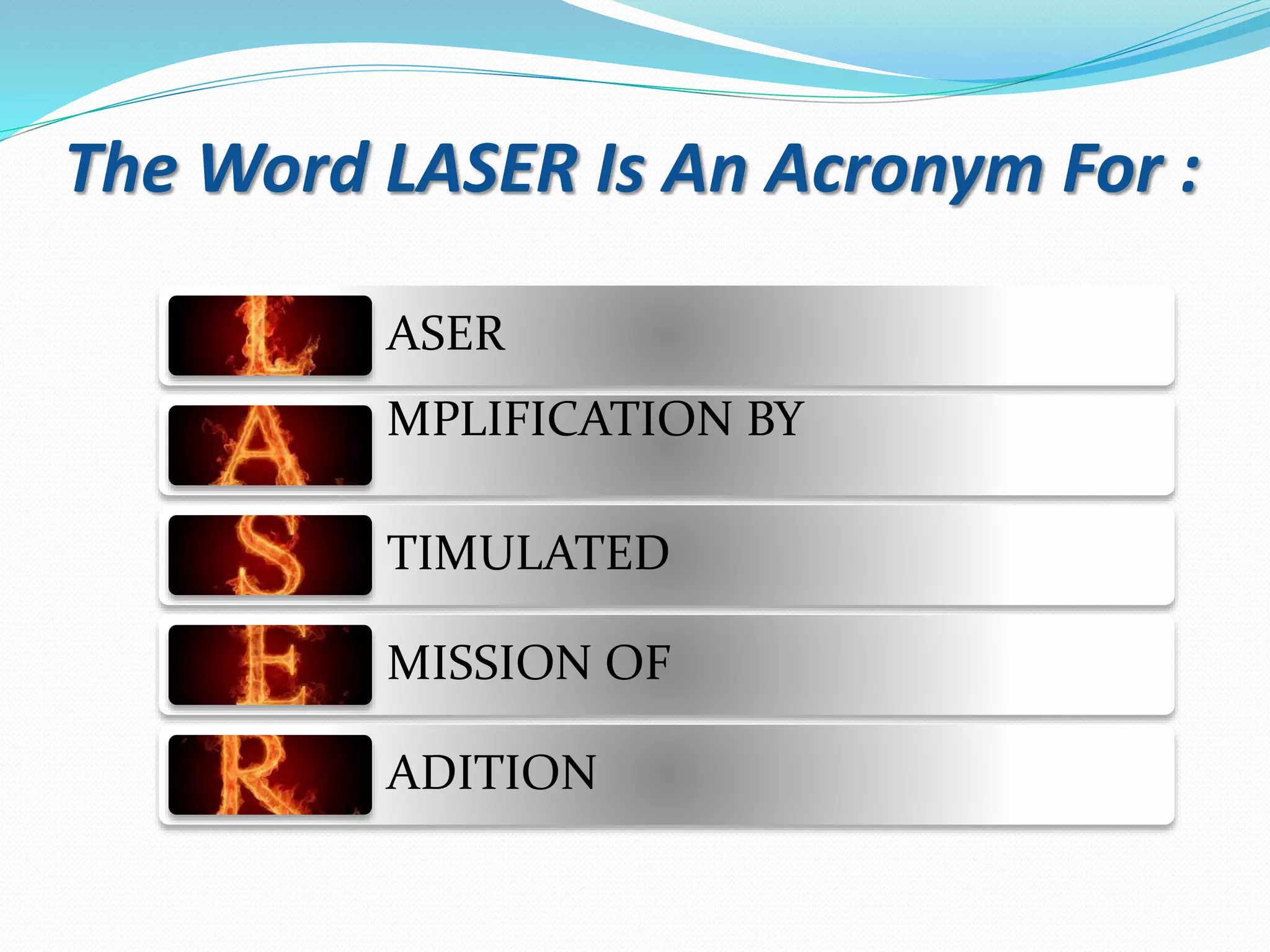
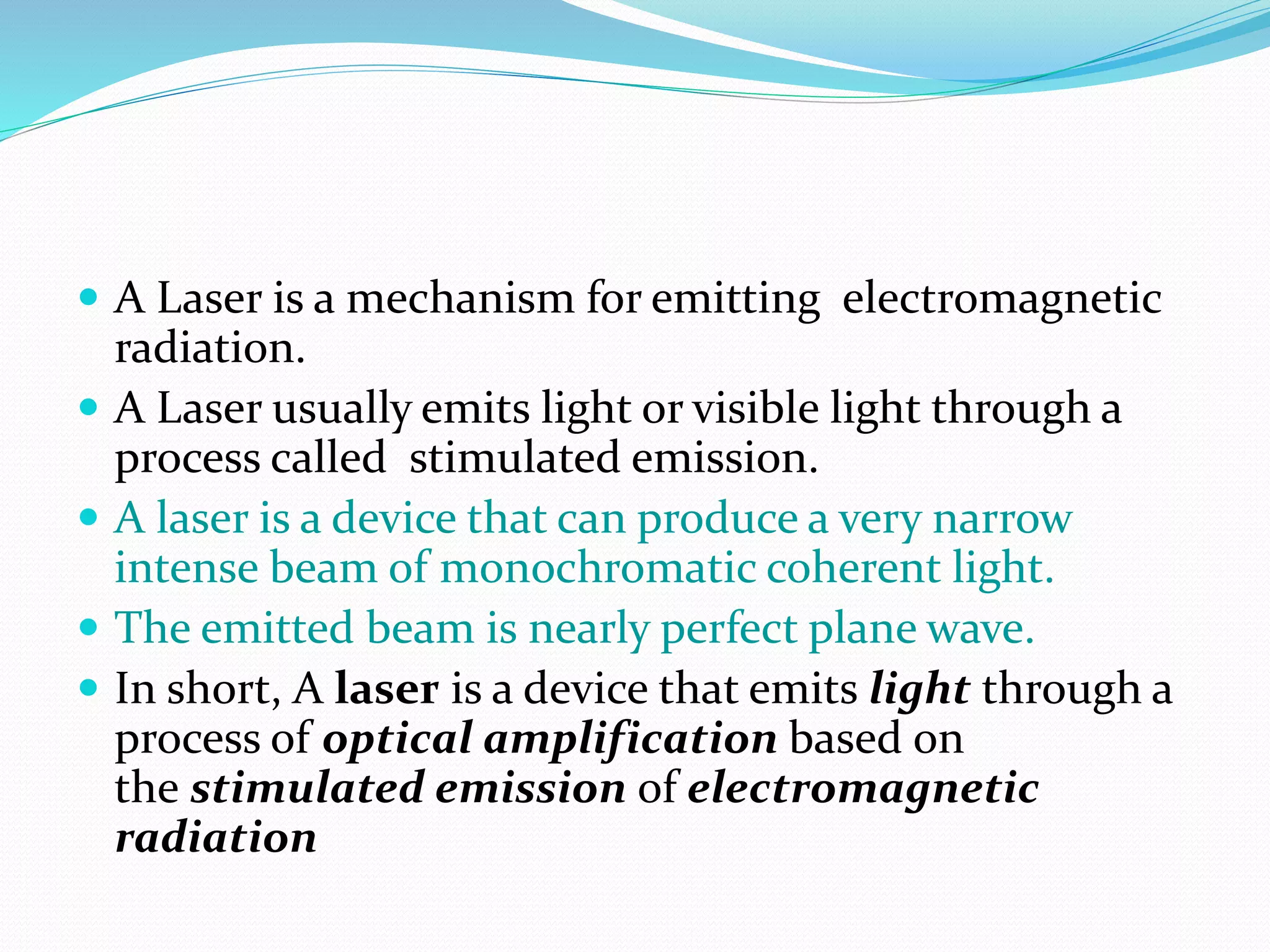
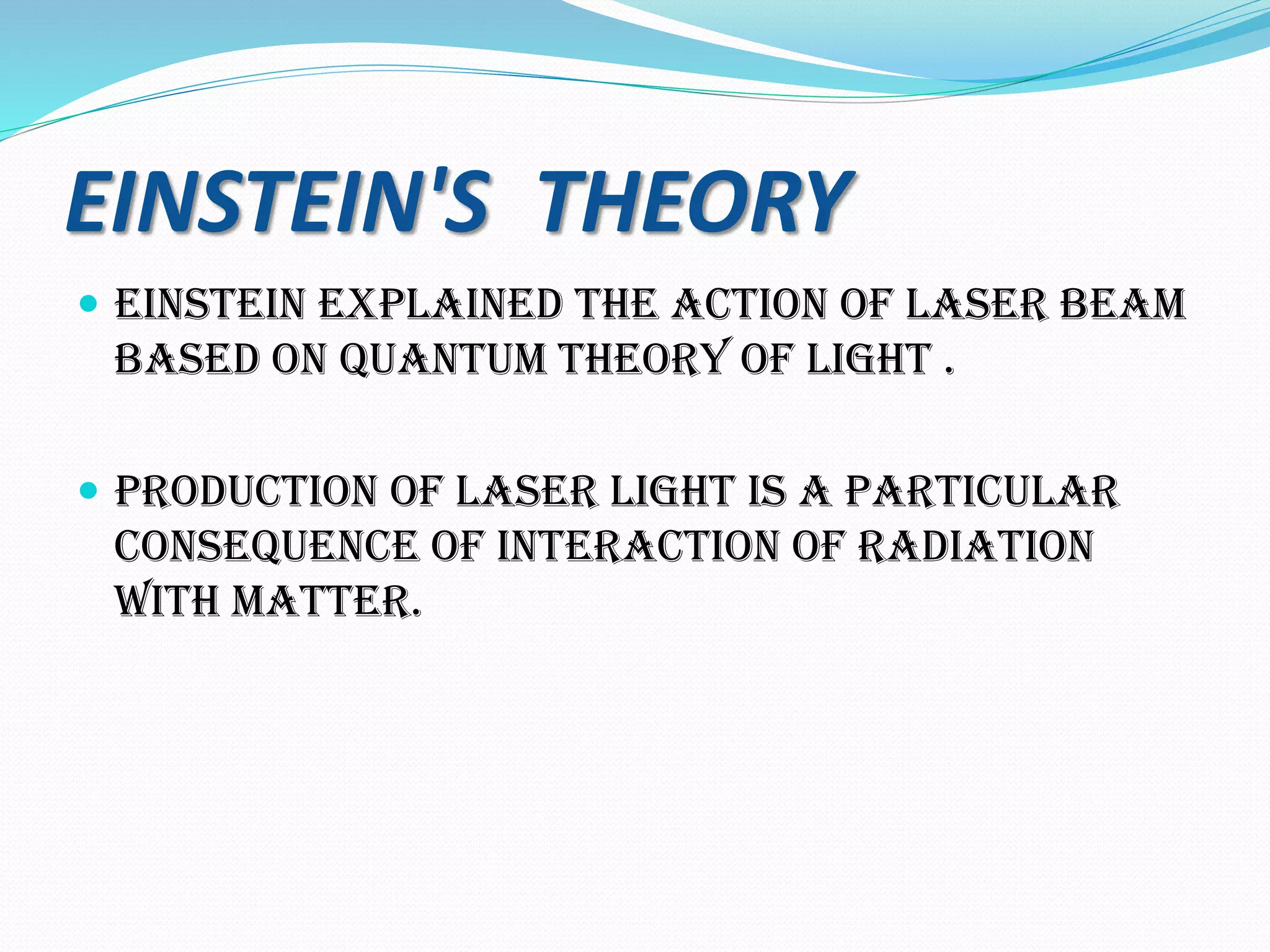
The document discusses lasers, providing details on:
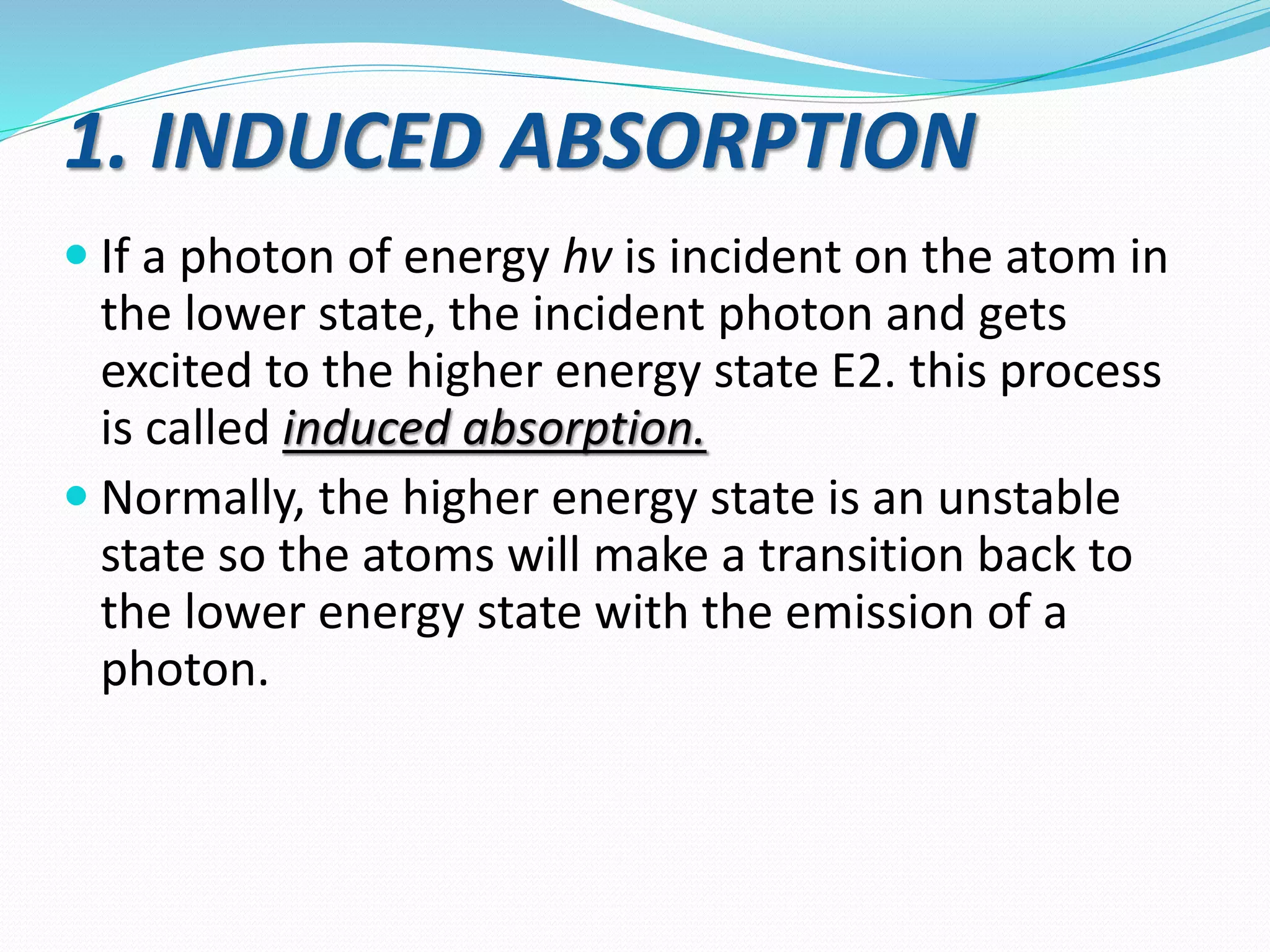
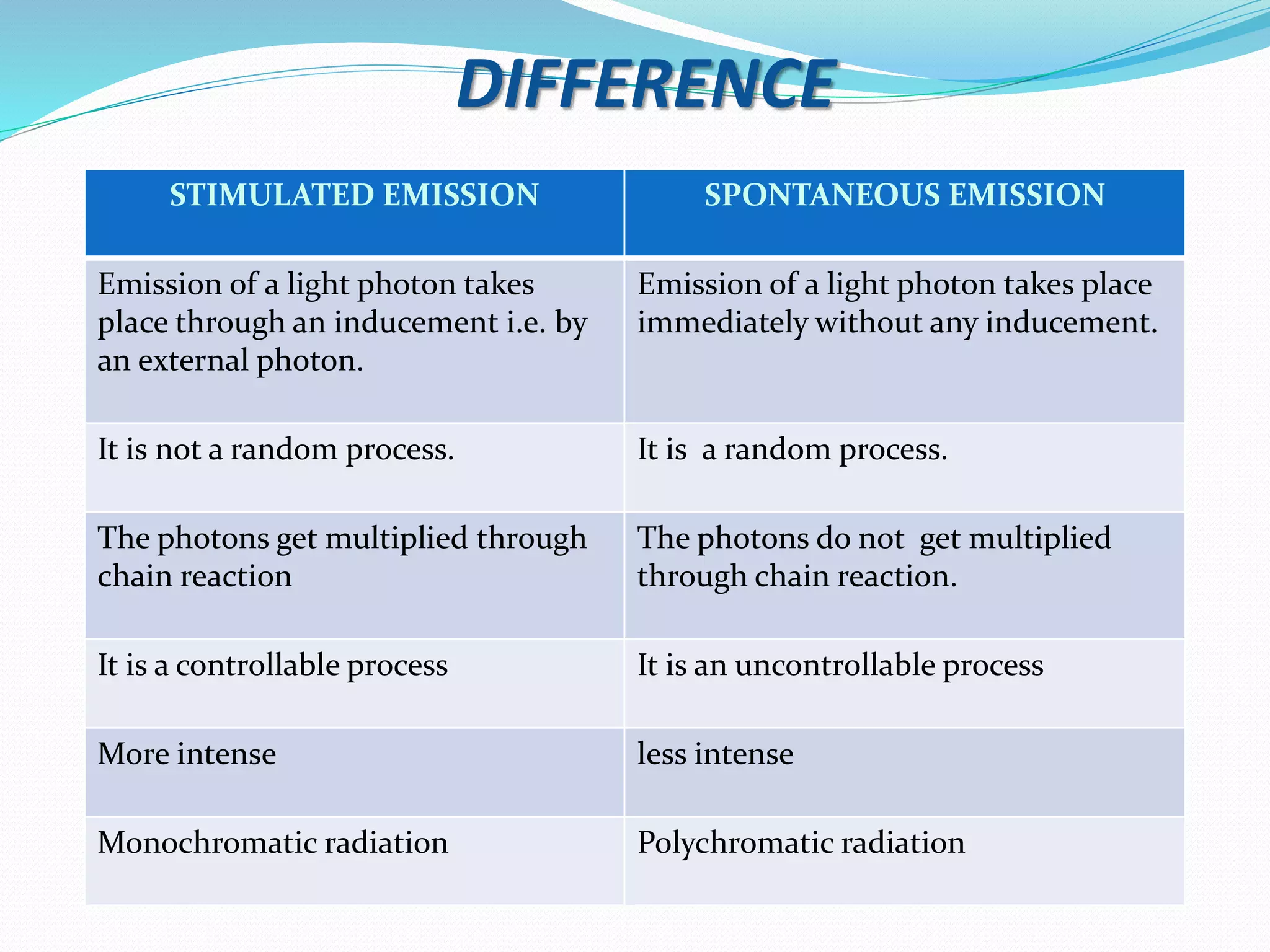
1. How lasers work through the process of stimulated emission of radiation, using a pumping mechanism to create population inversion in the active medium.
2. The key characteristics of laser light being monochromatic, coherent, and highly directional.
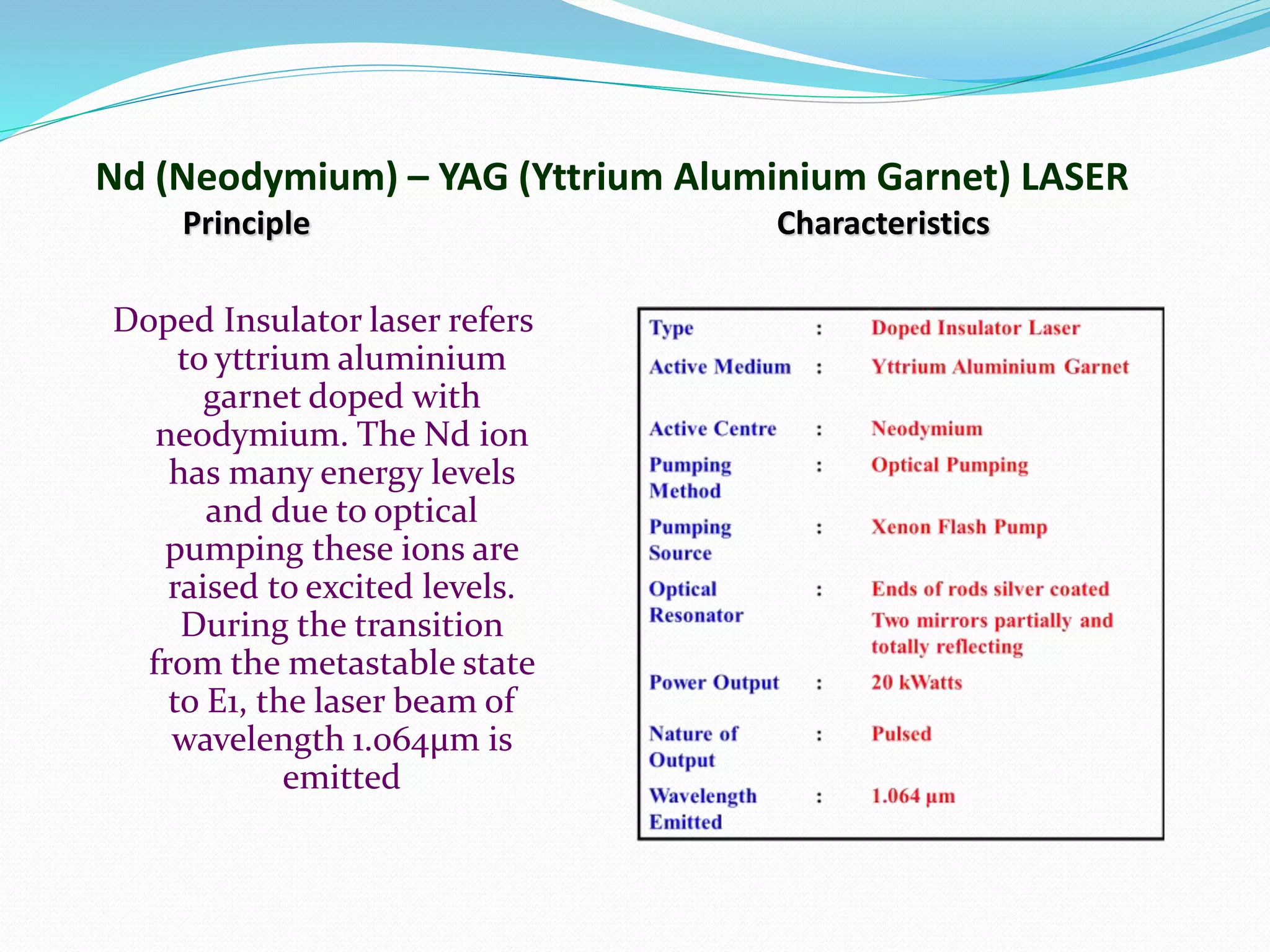
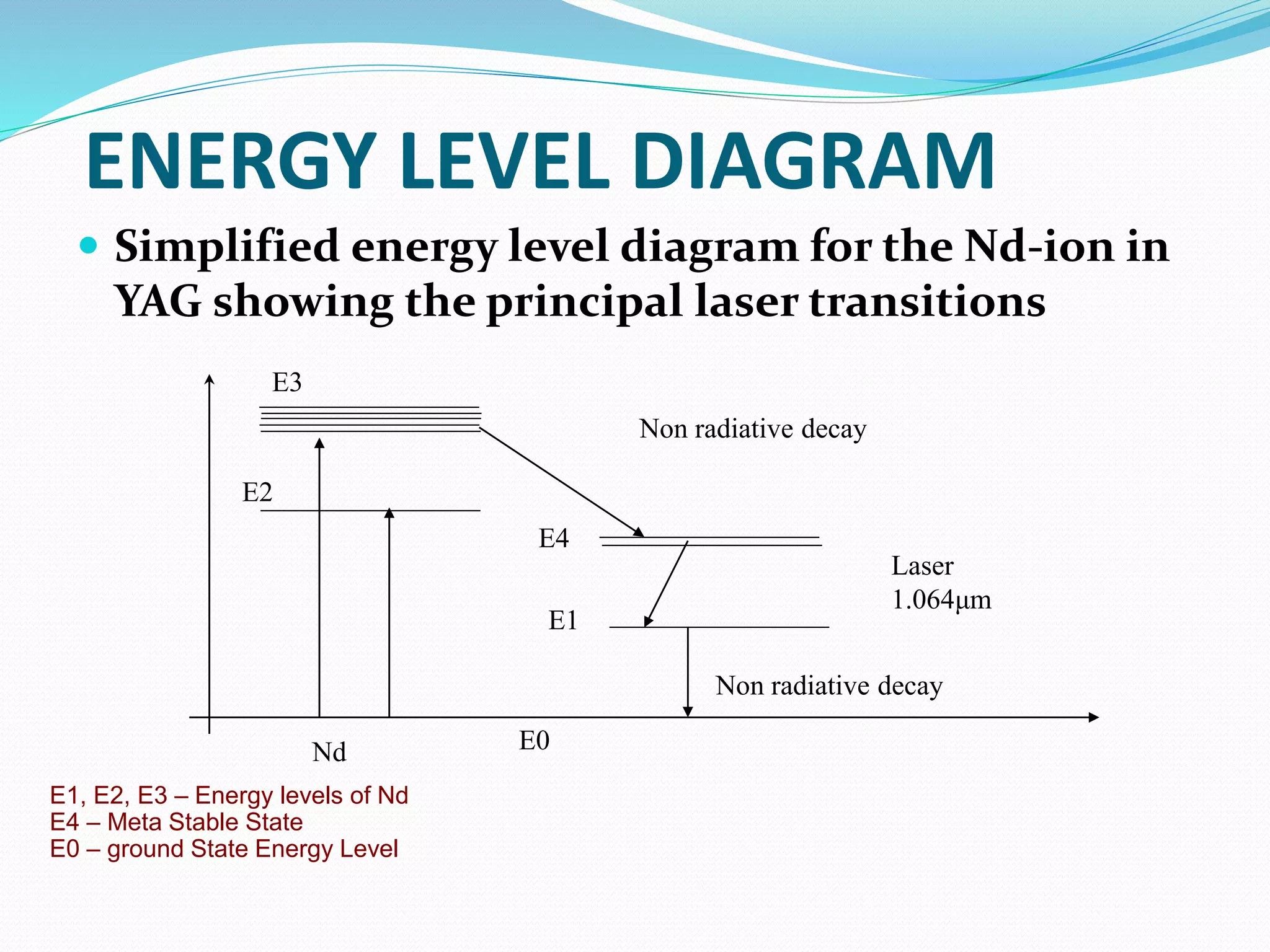
3. Examples of common laser types like Ruby and Nd:YAG lasers, describing their construction and working.
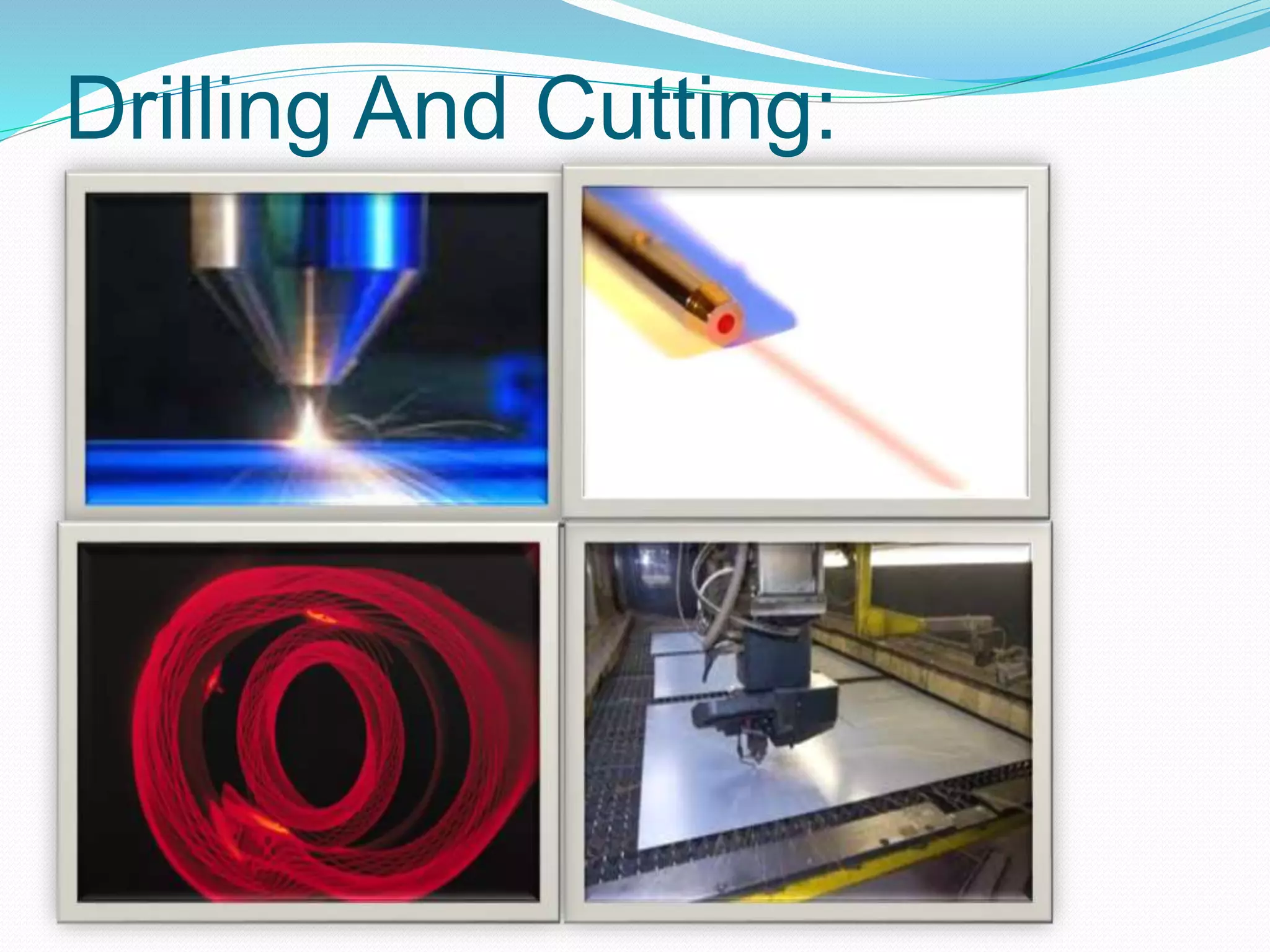
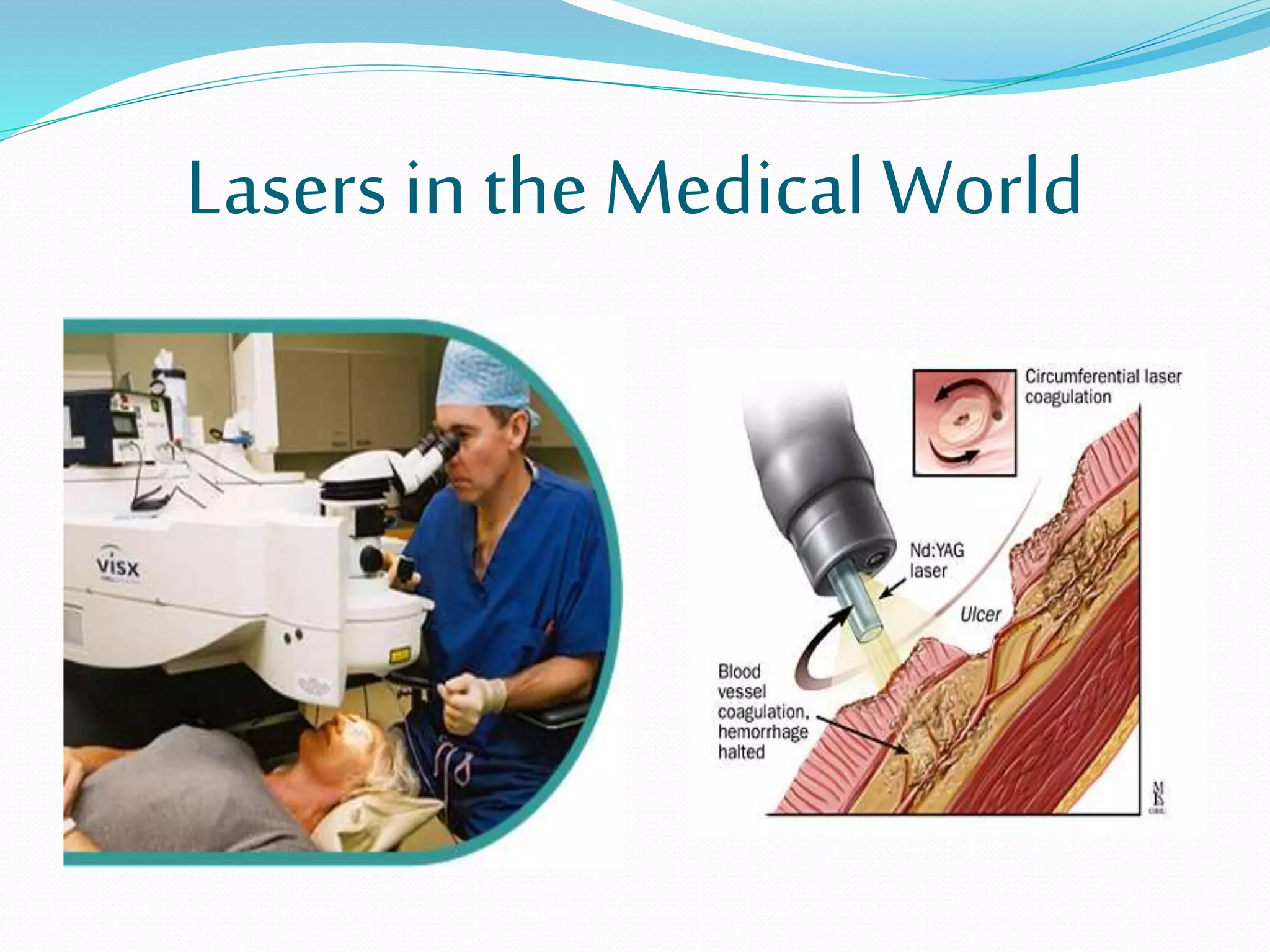
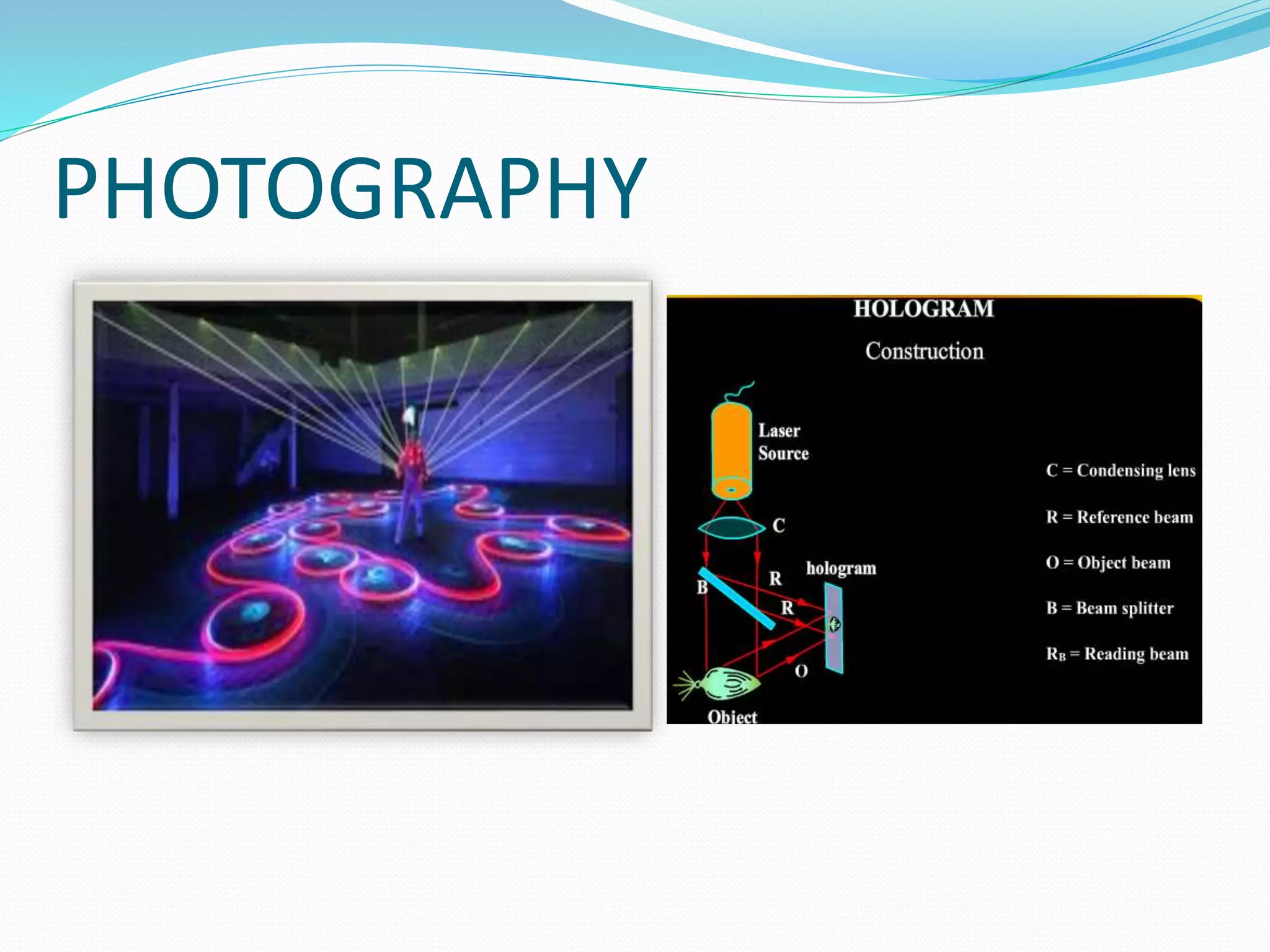
4. Applications of lasers in various fields like industry, medicine, communication, and more.