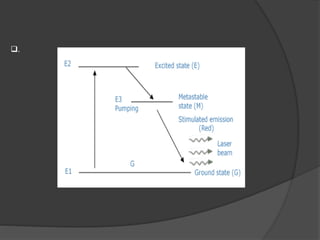
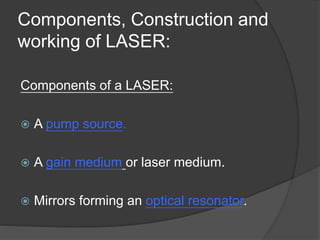
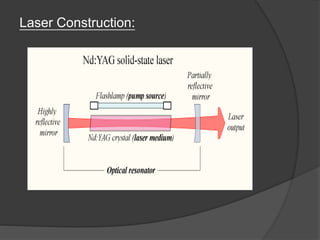
The document provides an overview of lasers, detailing their properties, components, and types, including solid-state, gas, liquid, excimer, chemical, and semiconductor lasers. It explains basic concepts such as absorption, spontaneous emission, stimulated emission, and population inversion, which are essential for laser operation. Furthermore, it outlines various applications of lasers in medicine, security, industry, and communication.