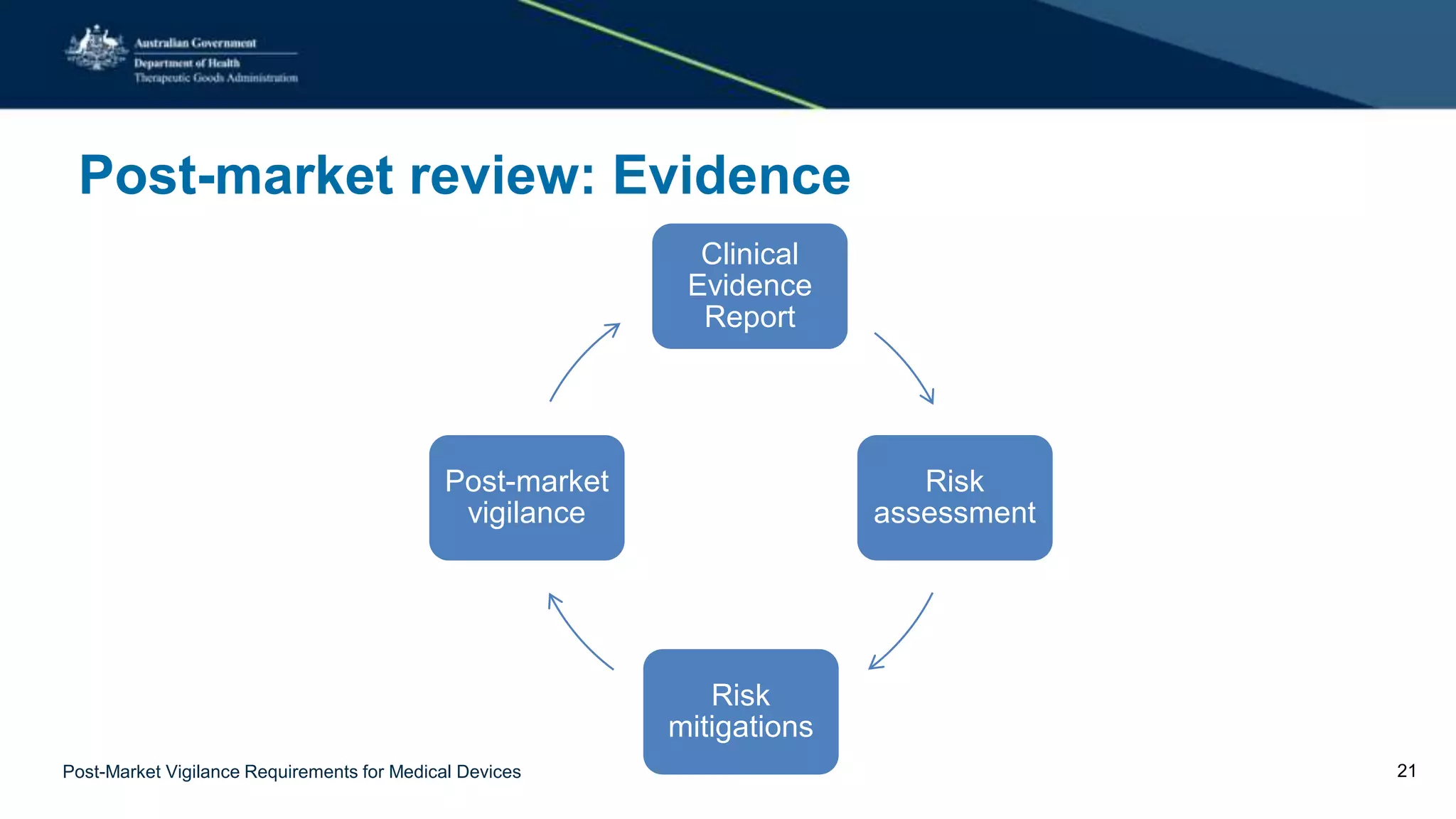
The document outlines the Therapeutic Goods Administration's (TGA) post-market vigilance requirements for medical devices, emphasizing its role in ensuring safety and compliance through regulatory oversight. It provides definitions for vigilance and surveillance, describes the risk-based approach to regulation, and outlines sponsors' obligations for reporting adverse events. Additionally, it details the process for investigating incidents, the outcomes of reviews, and the necessity for continuous compliance monitoring to protect public health.