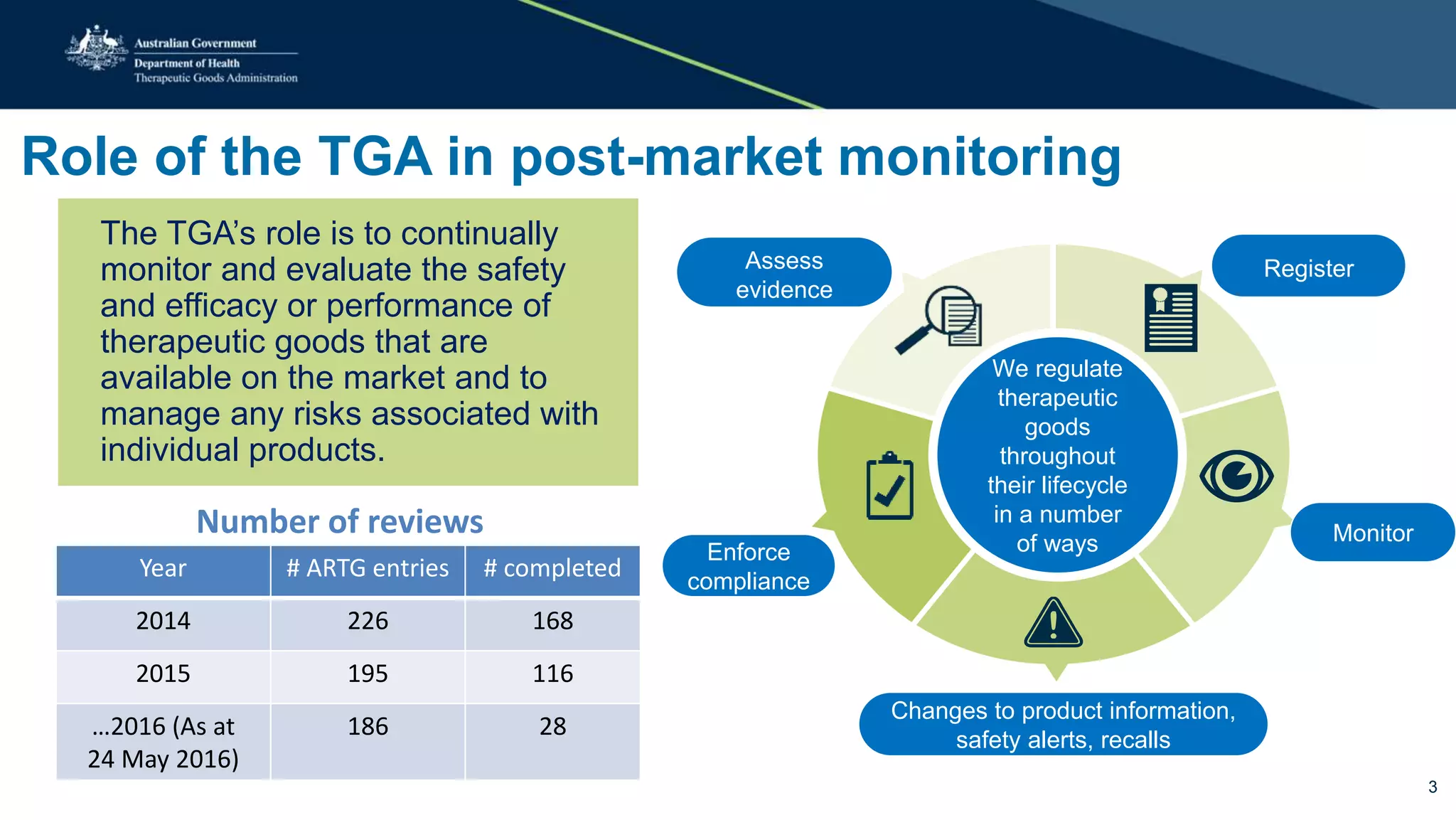
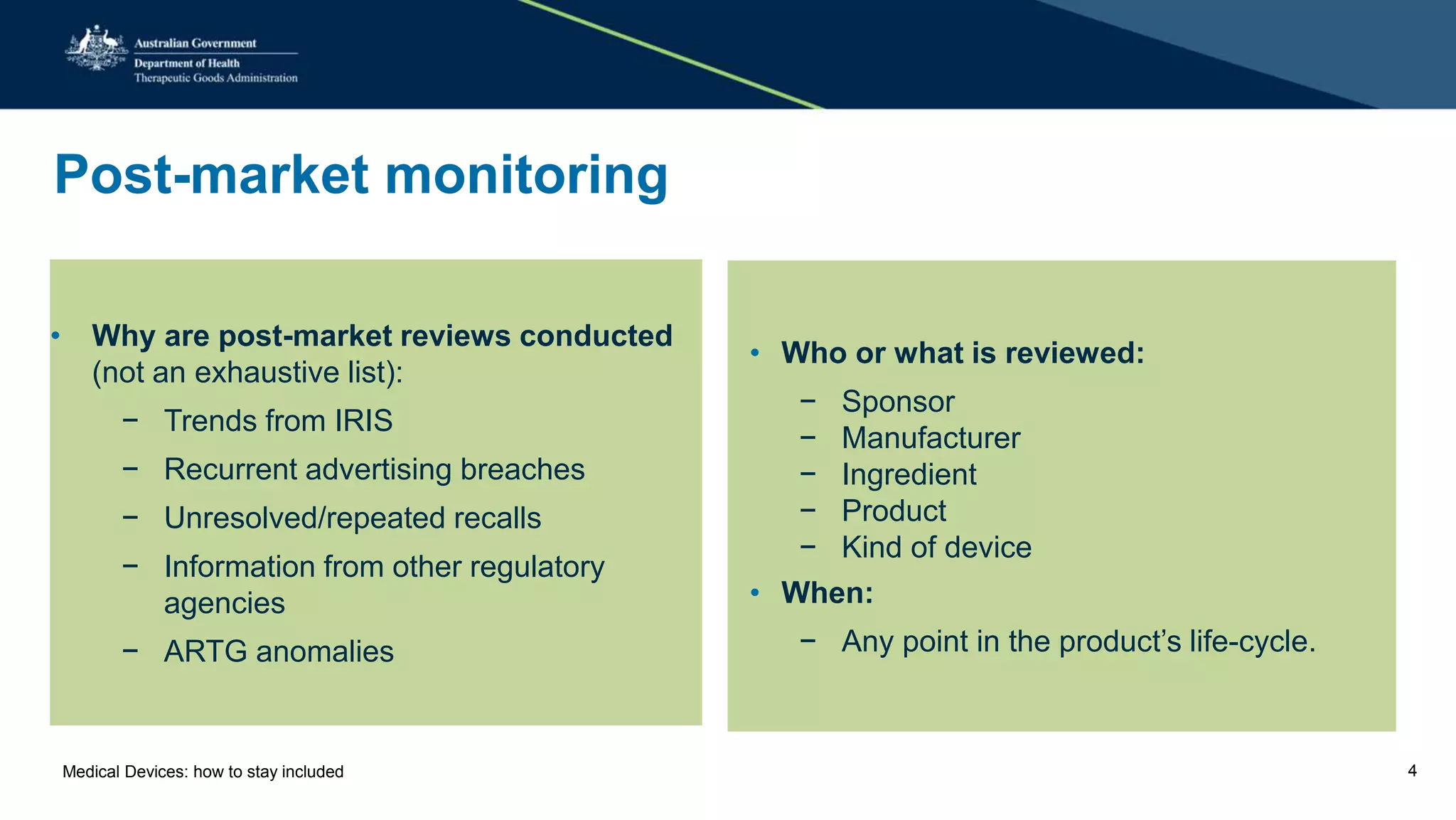
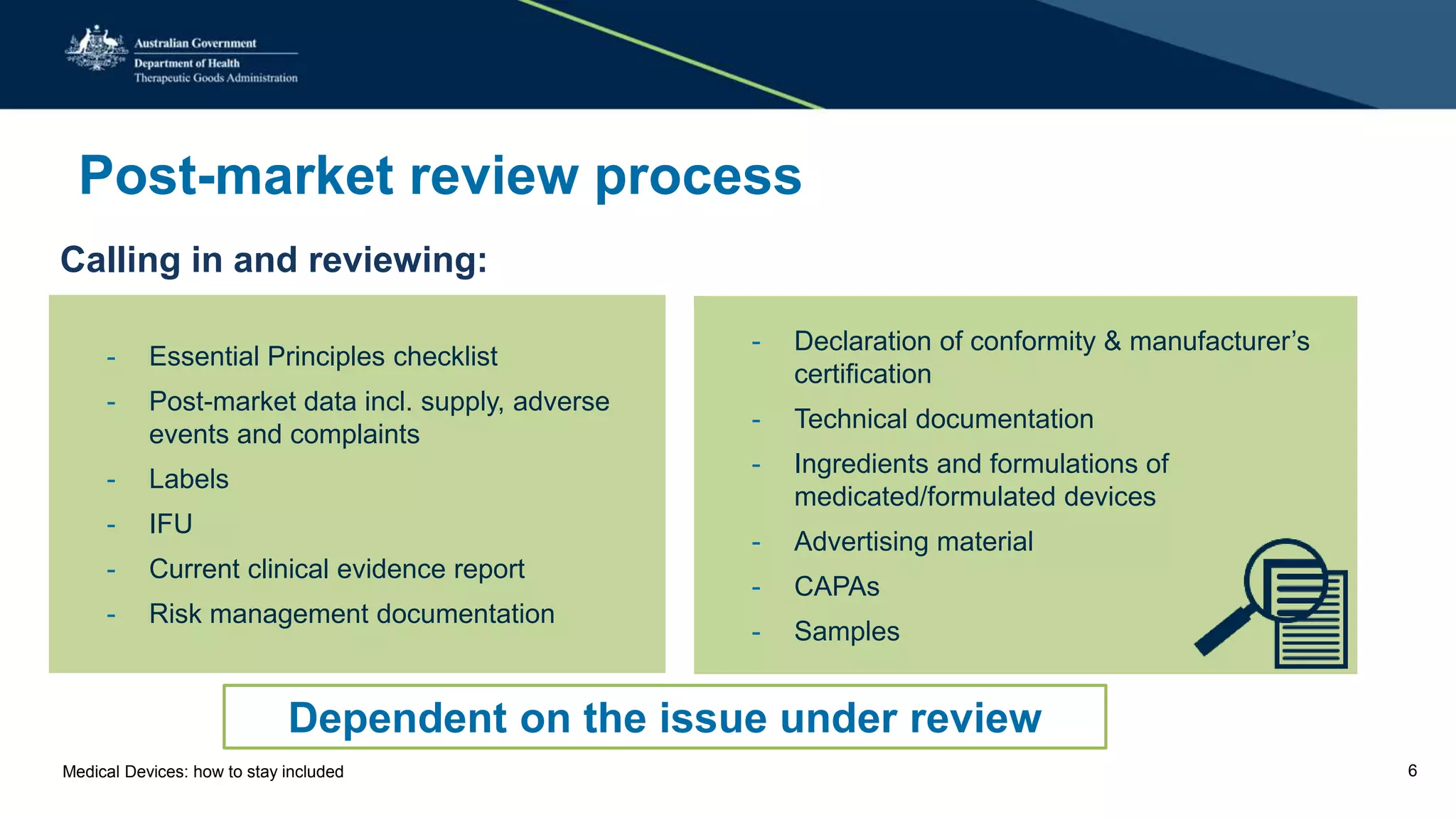
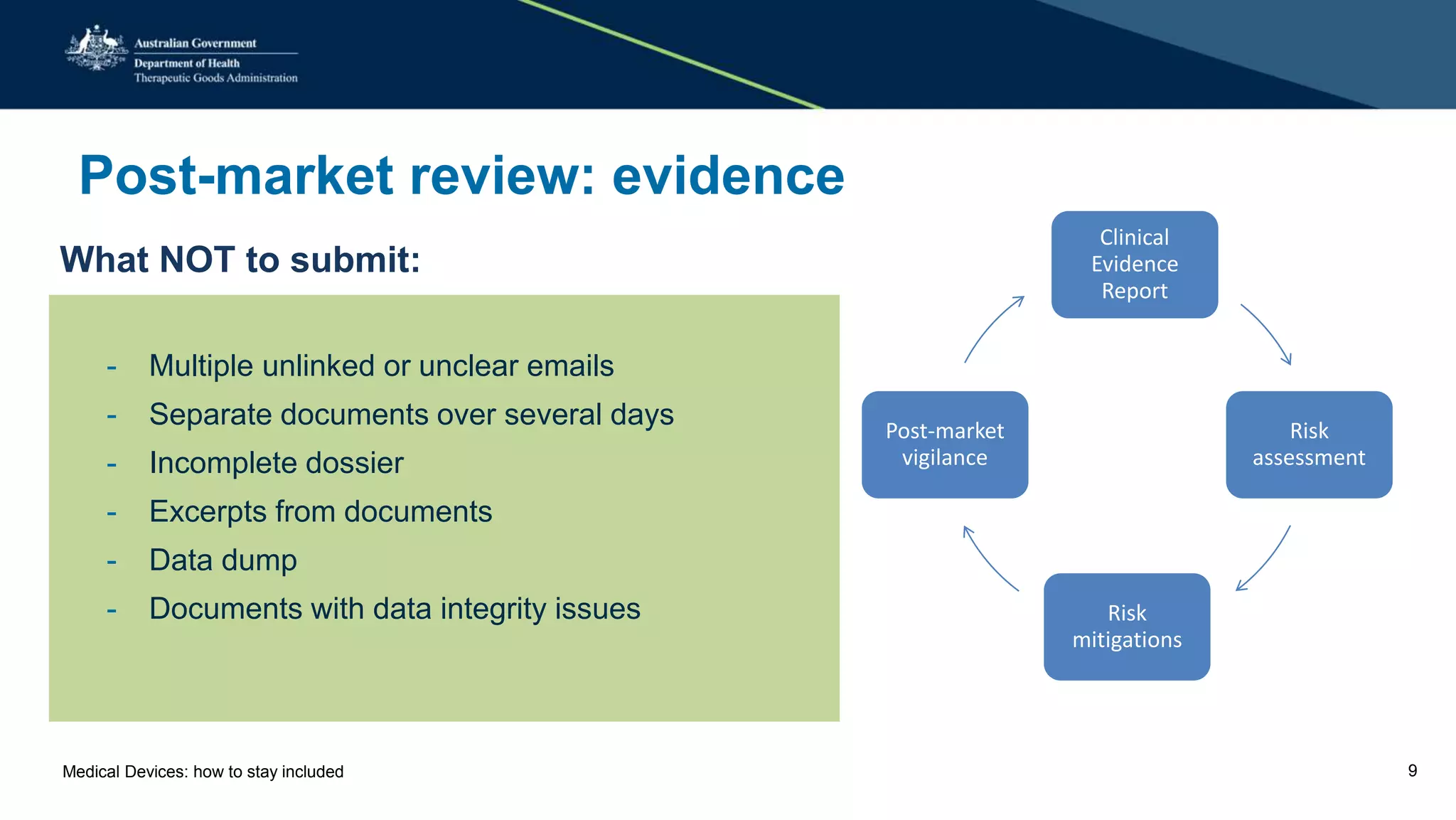
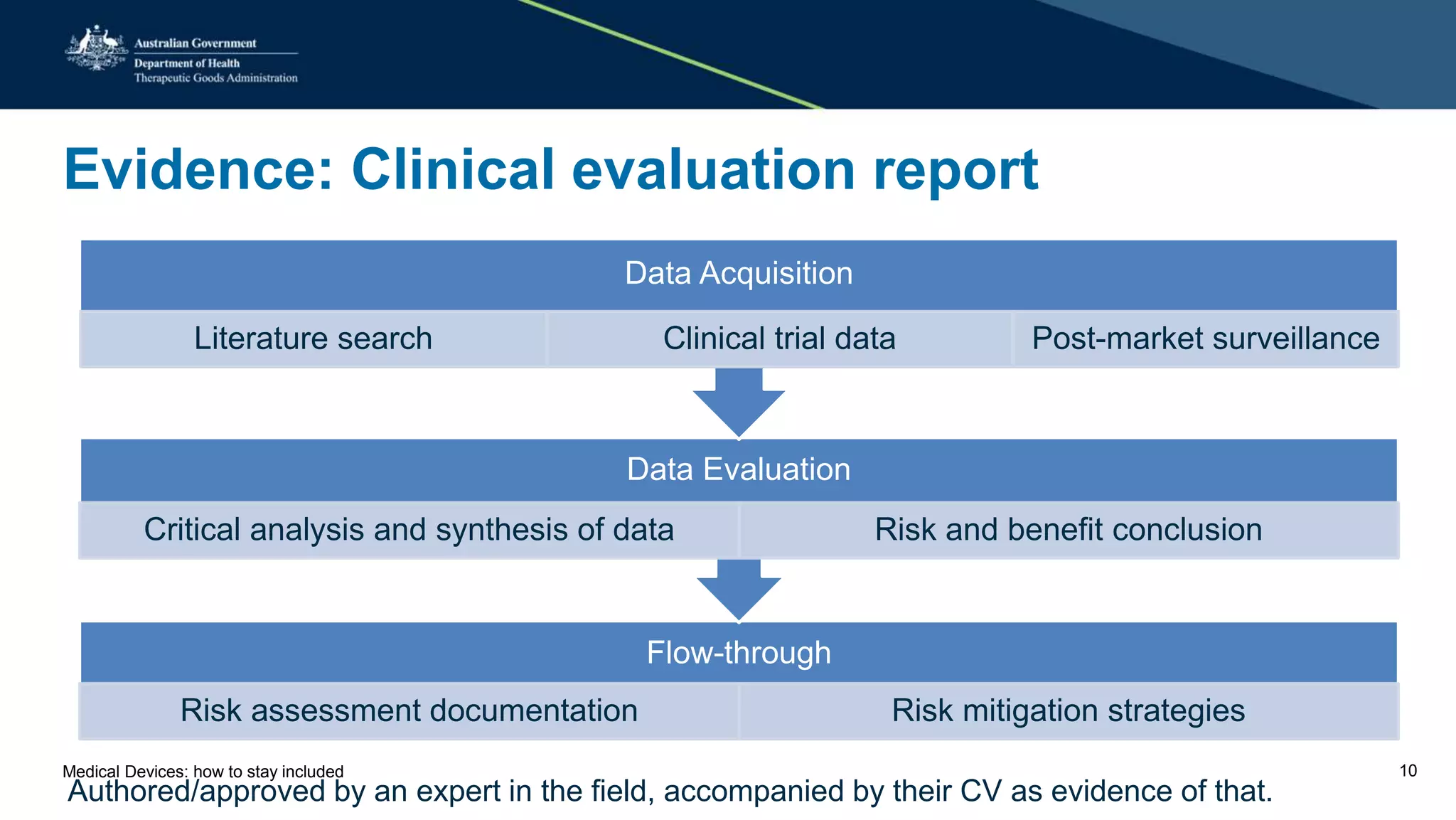
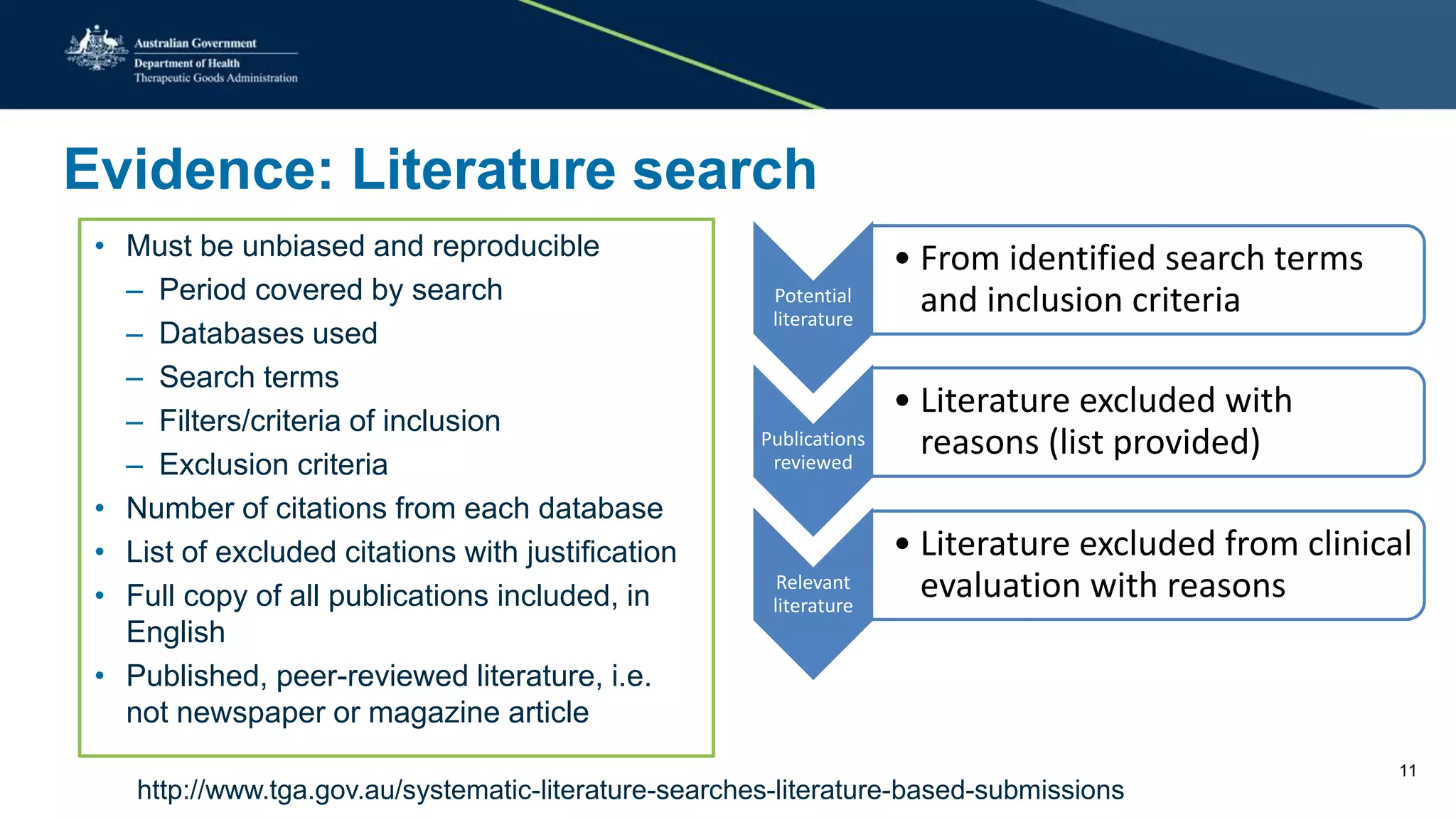
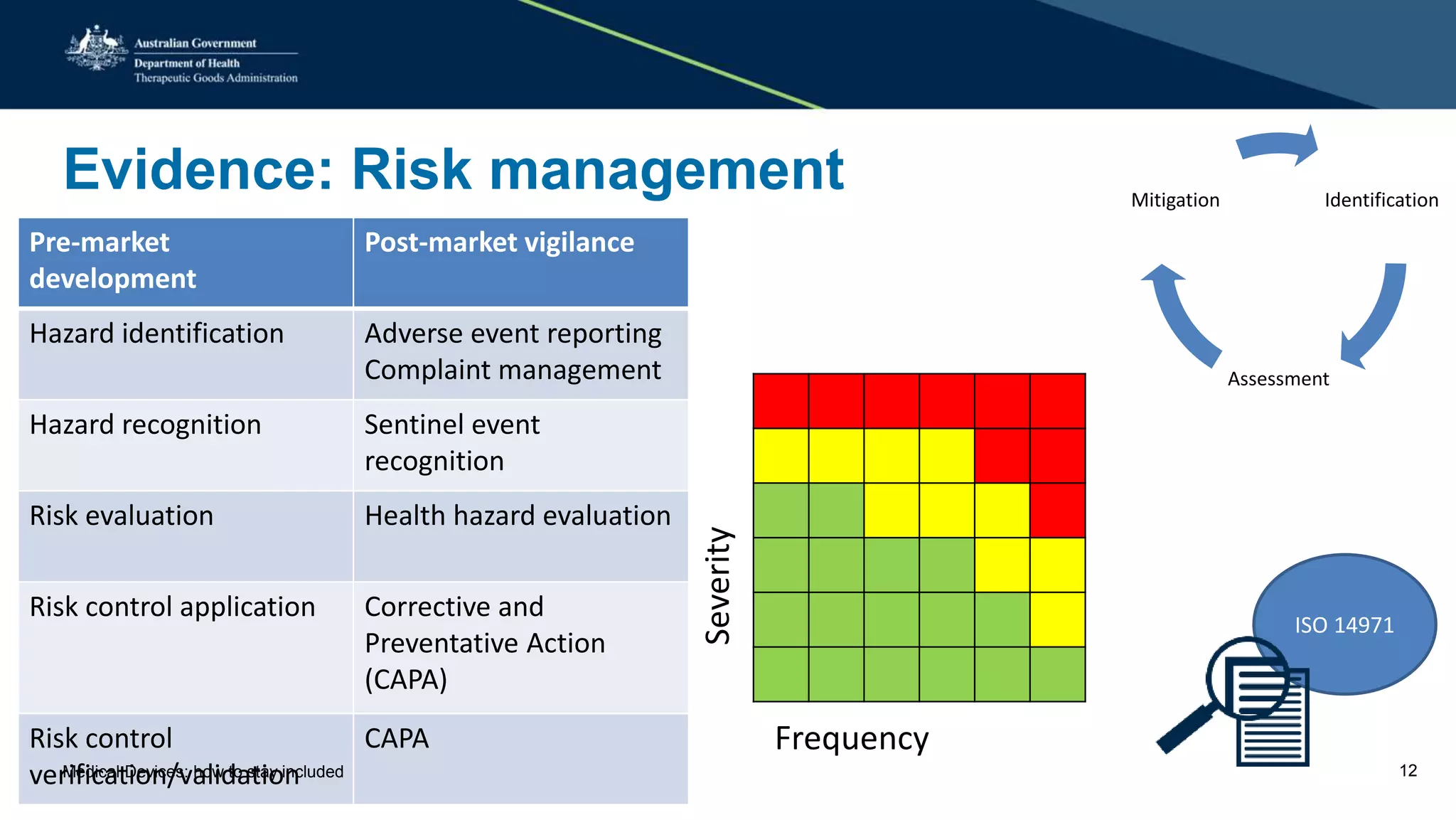
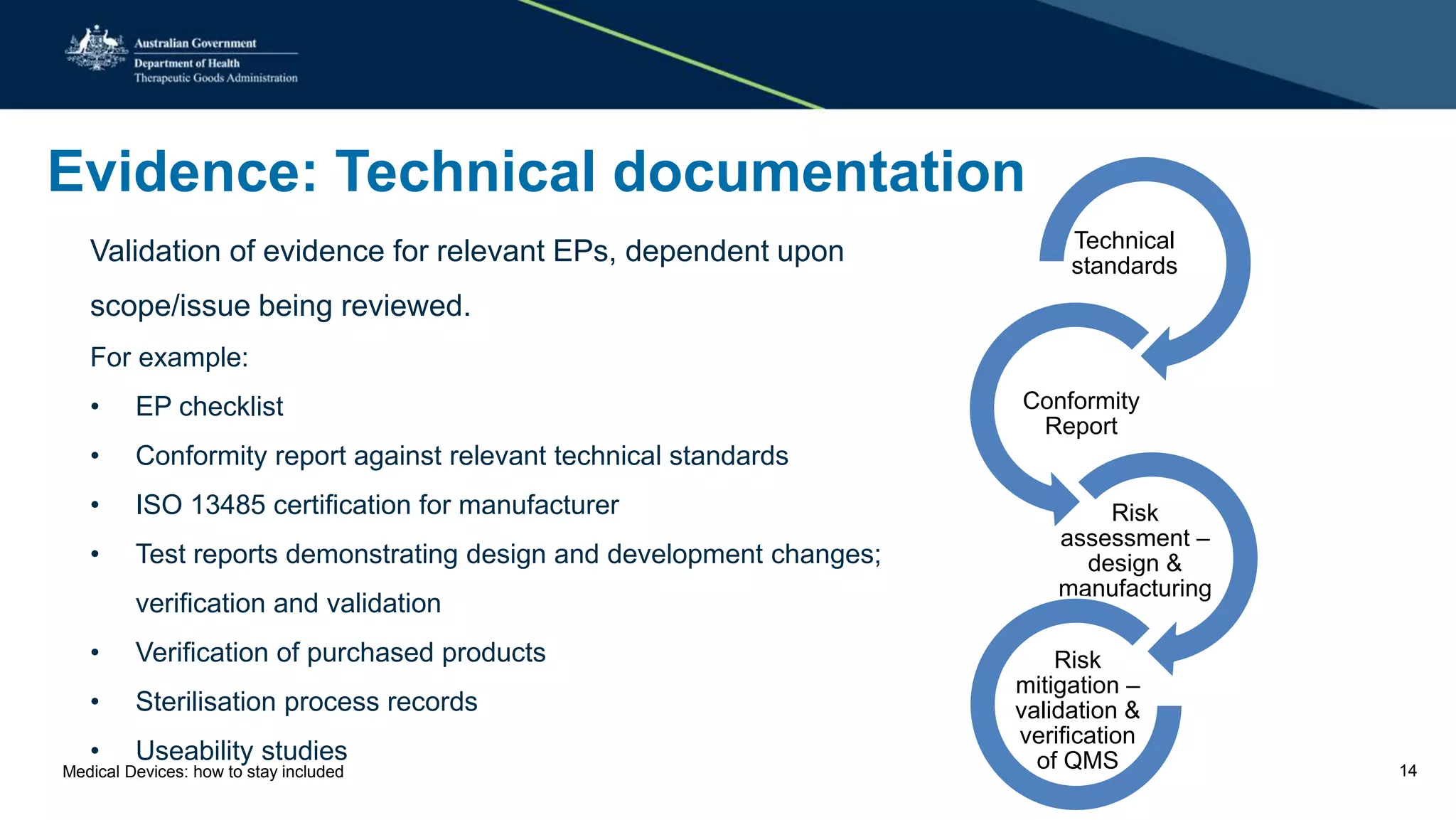
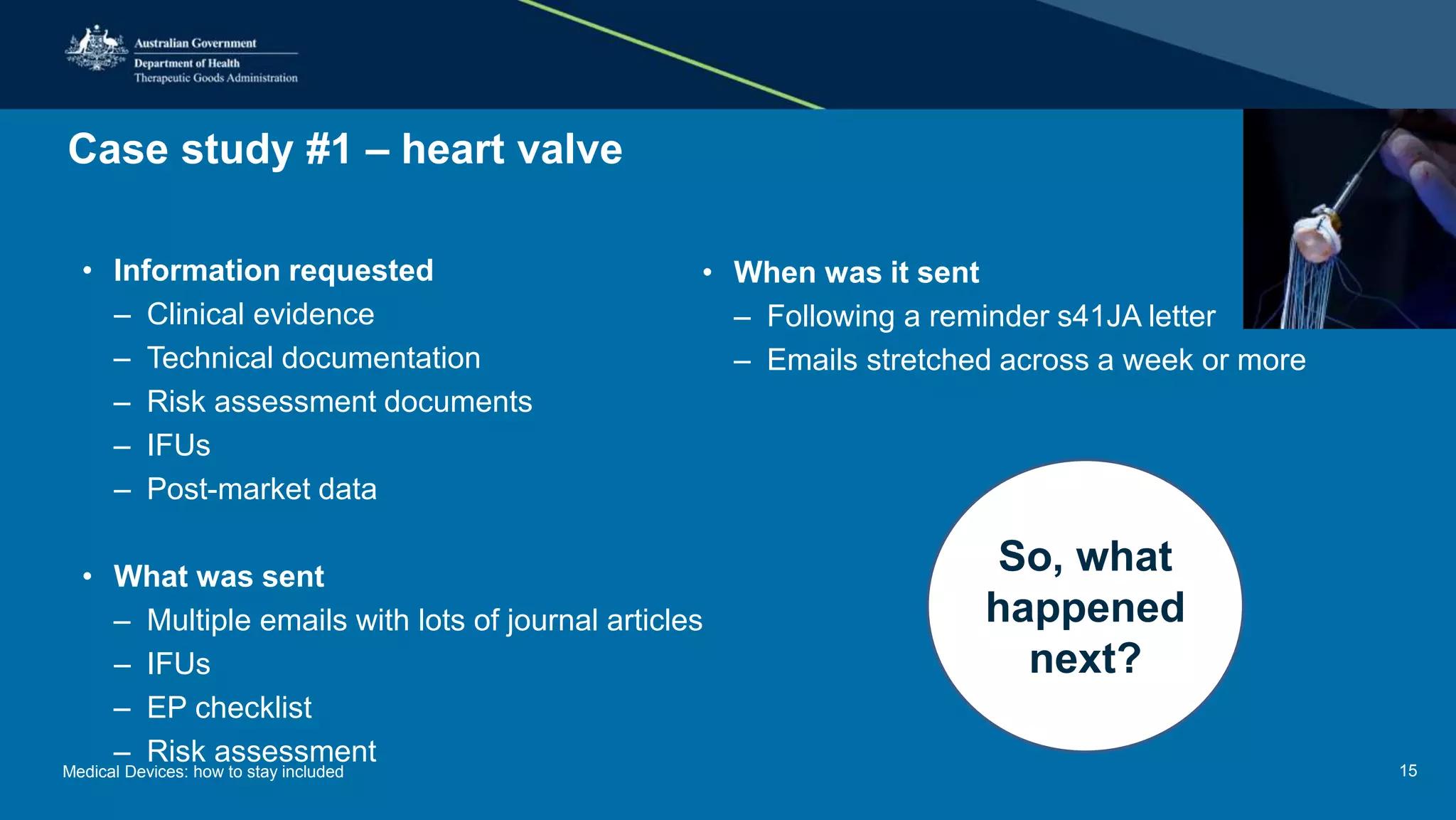
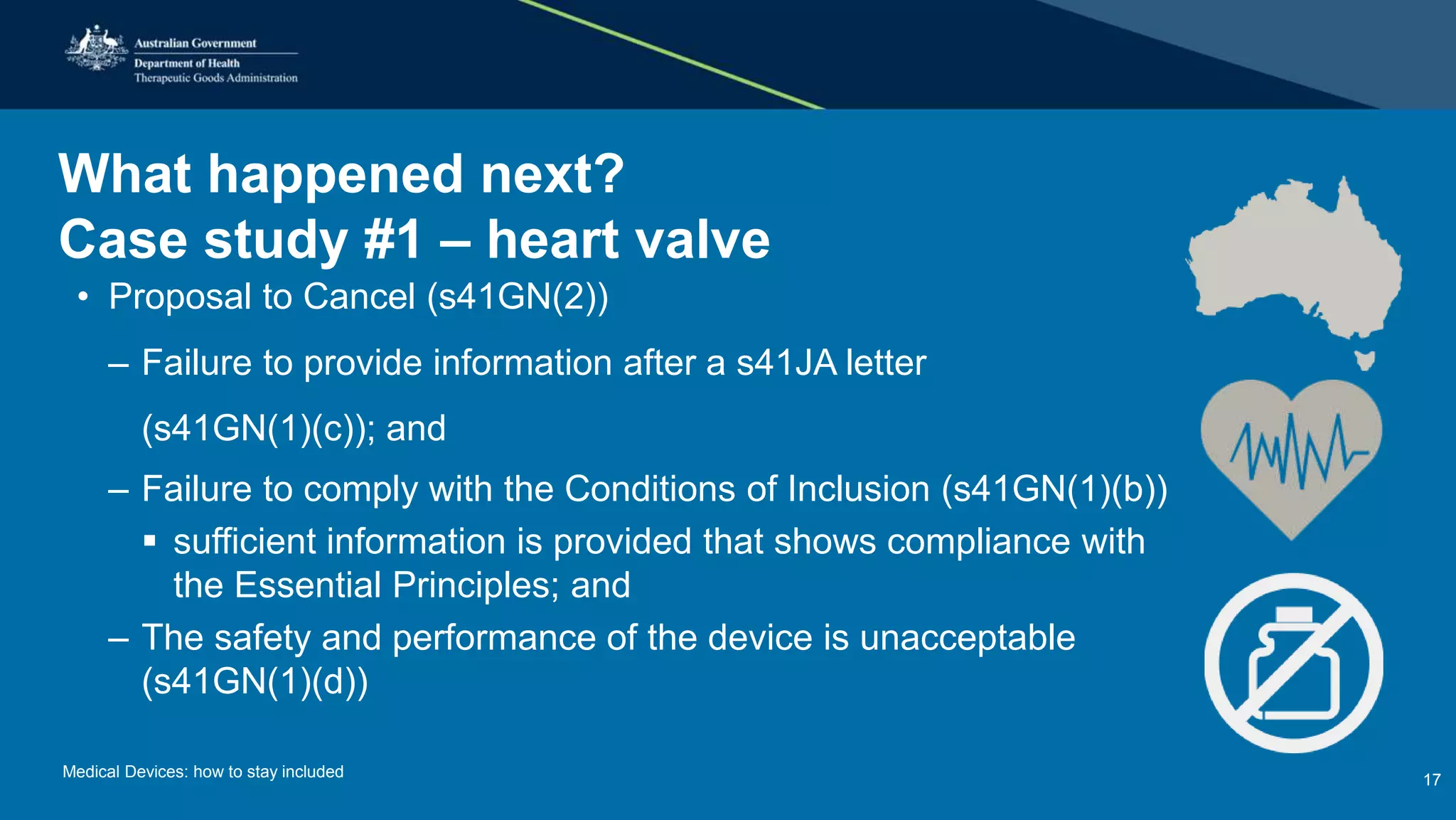
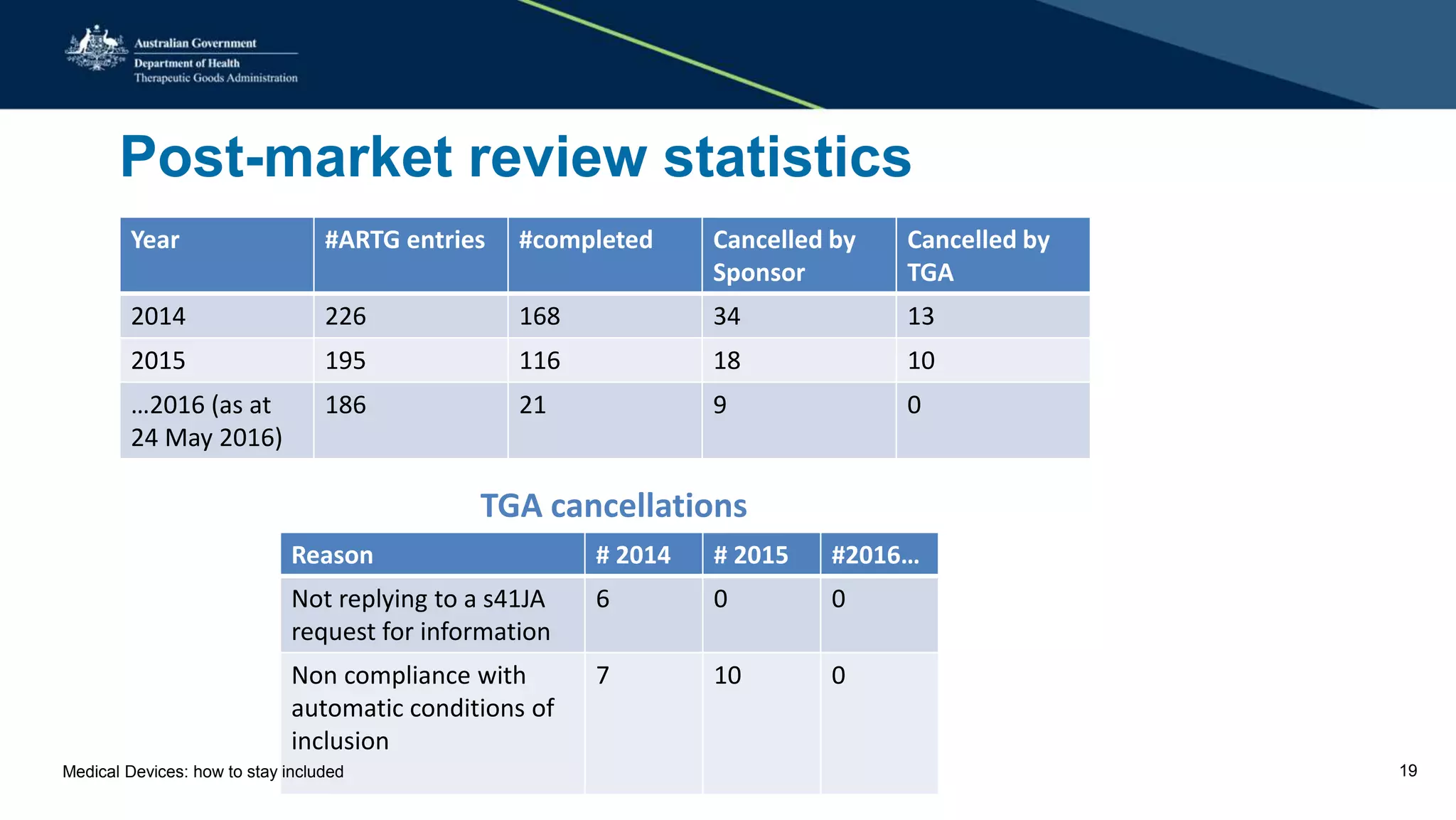
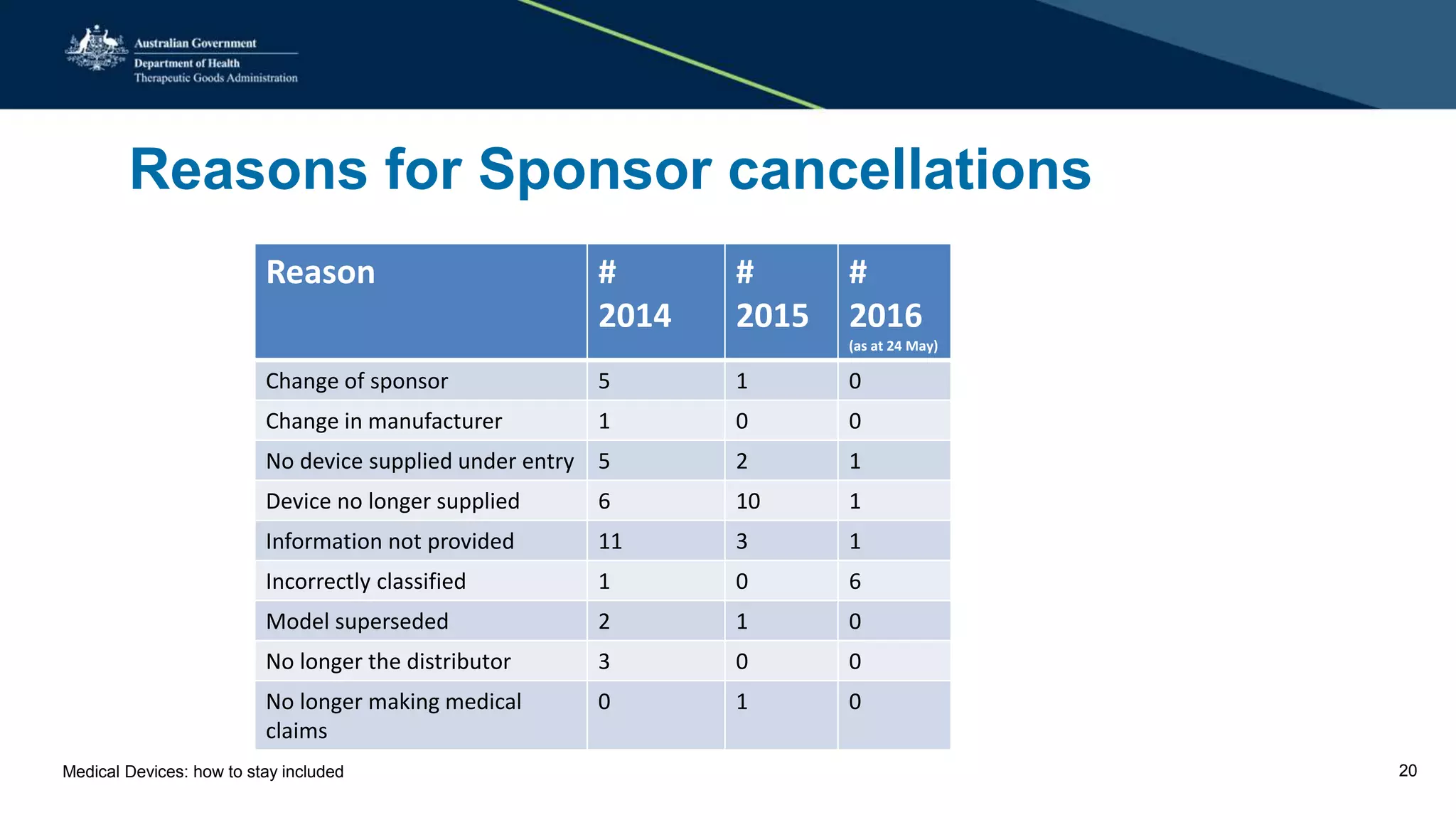
The document outlines the post-market review process for medical devices managed by the TGA, emphasizing the need for strong collaboration between manufacturers and sponsors, as well as the importance of proper documentation for compliance. It provides insights into monitoring, risk assessment, and the types of evidence required during reviews, including case studies on heart valves and tissue morcellators. Additionally, it highlights key messages for manufacturers on submission requirements and encourages adherence to established guidelines.