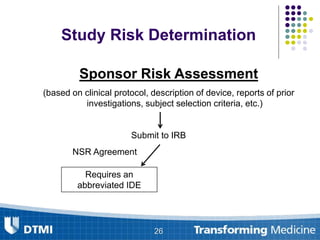
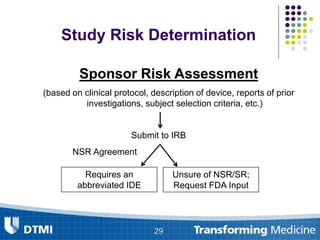
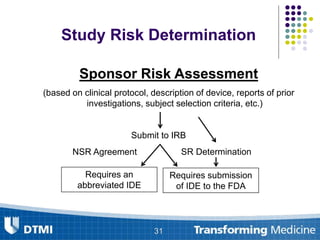
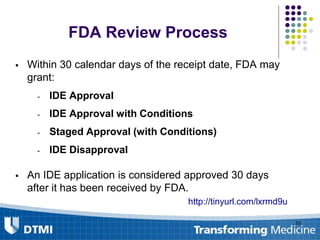
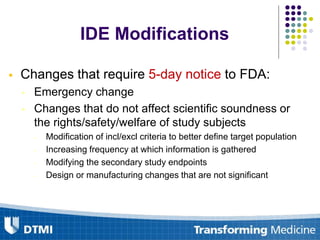
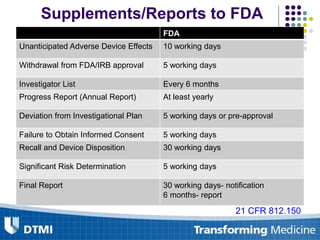
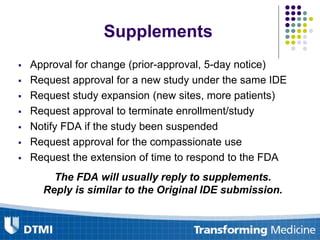
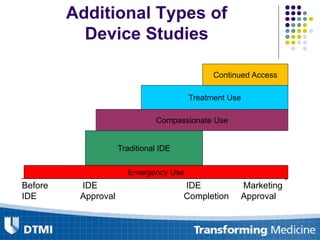
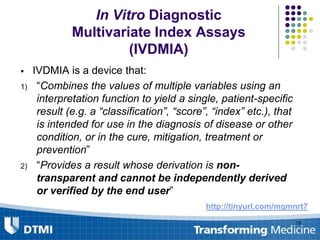
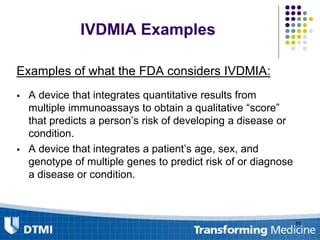
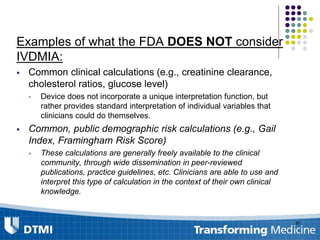
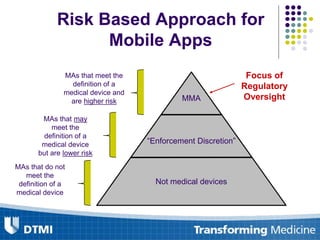
The document outlines best practices for preparing and maintaining Investigational Device Exemptions (IDE) for medical device studies, detailing definitions, clinical investigations, exemptions, risk determination, and submission processes. It emphasizes regulatory oversight by the FDA and includes guidelines for significant and non-significant risk device studies, investigation protocols, and required documentation. Additionally, it provides insights into the maintenance of active IDEs and modifications needed during research.