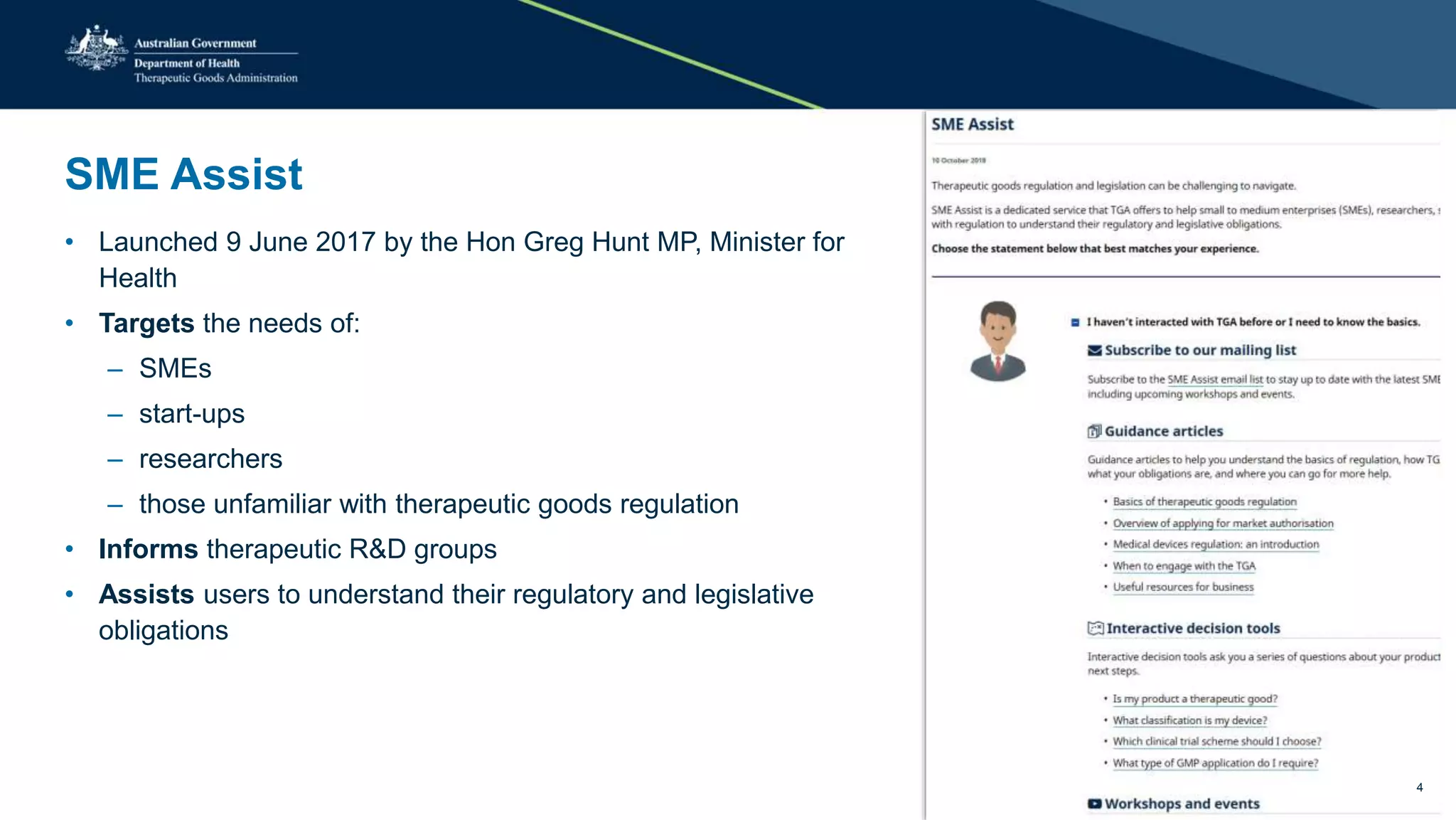
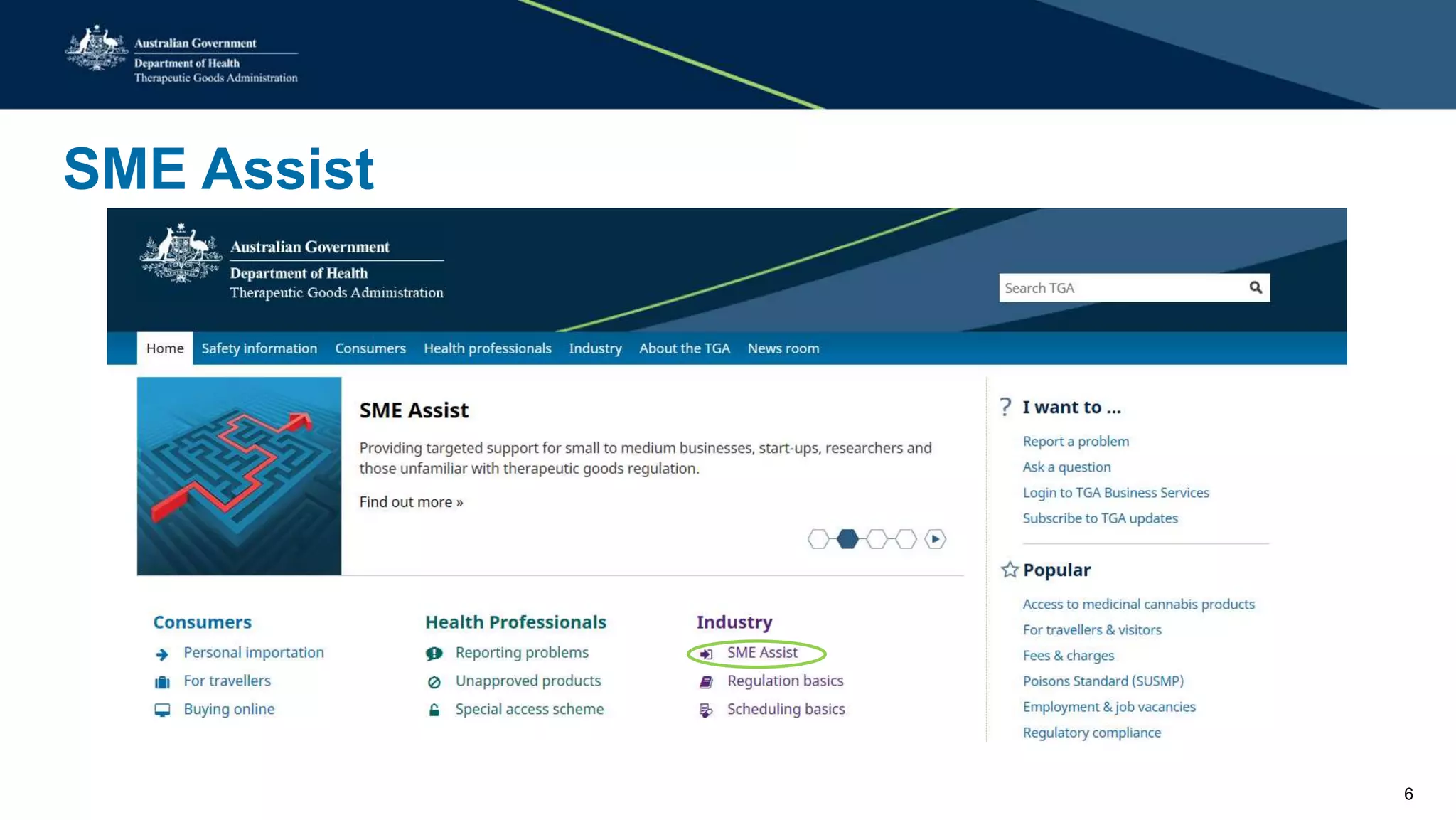
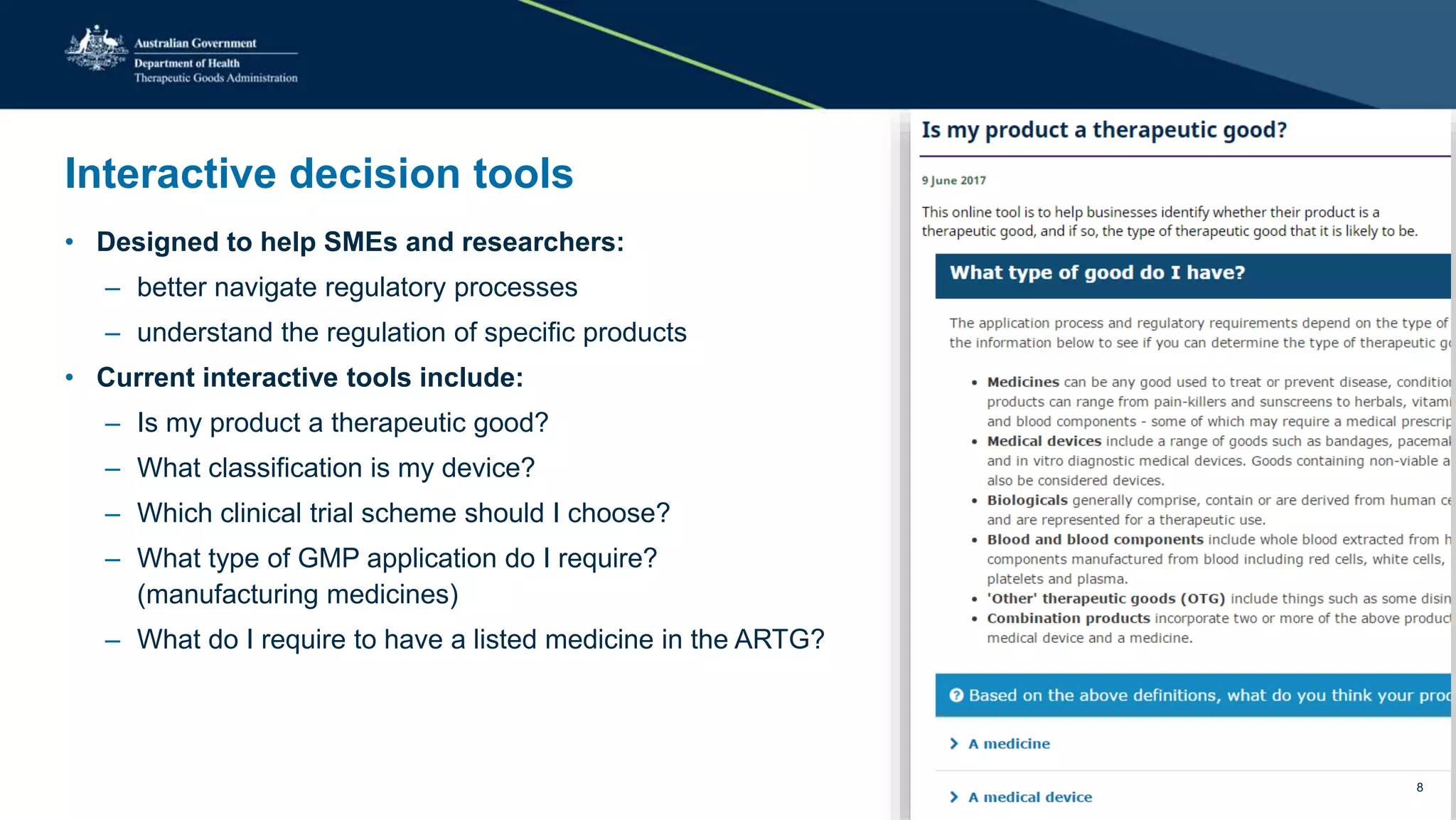
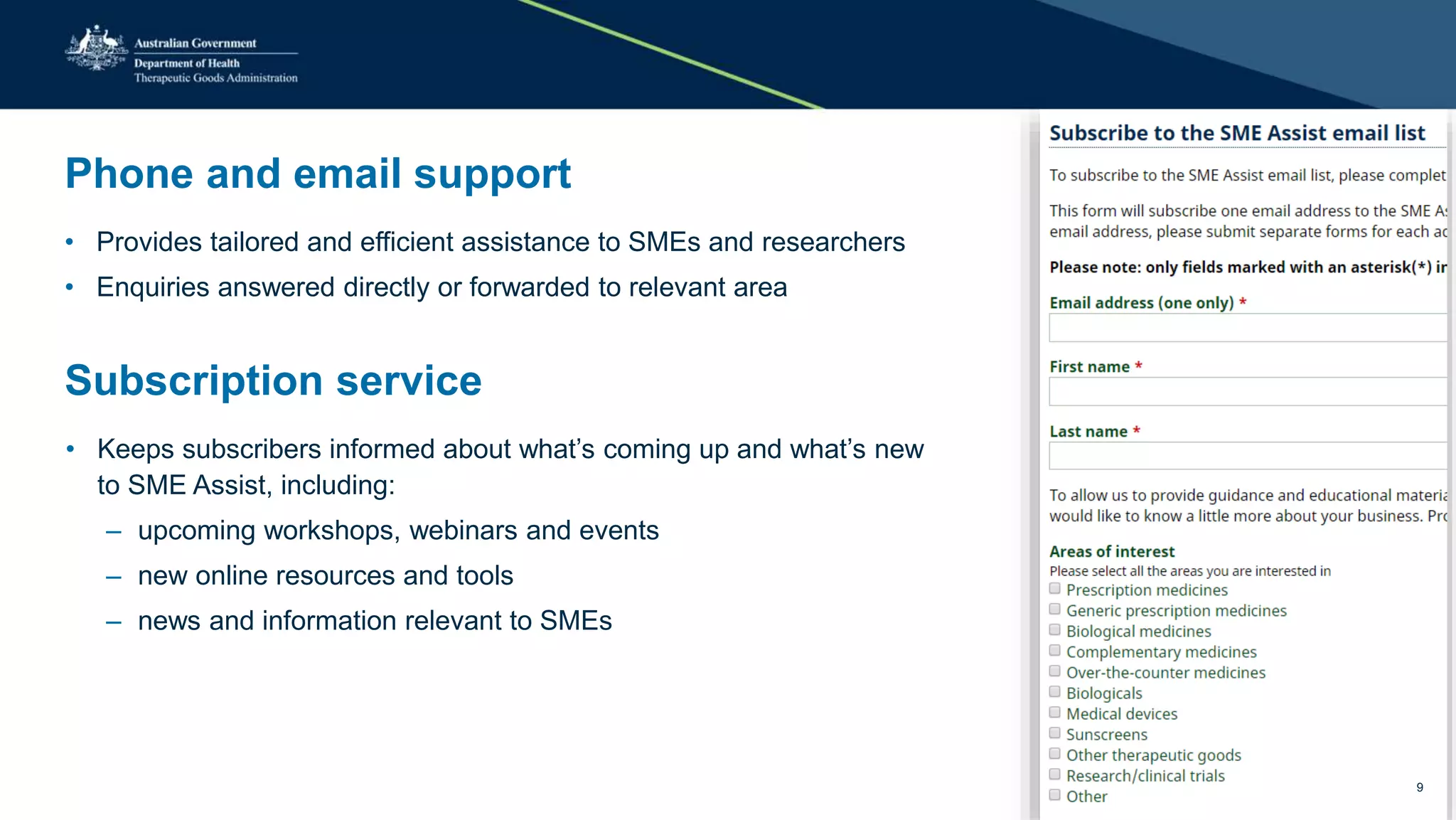
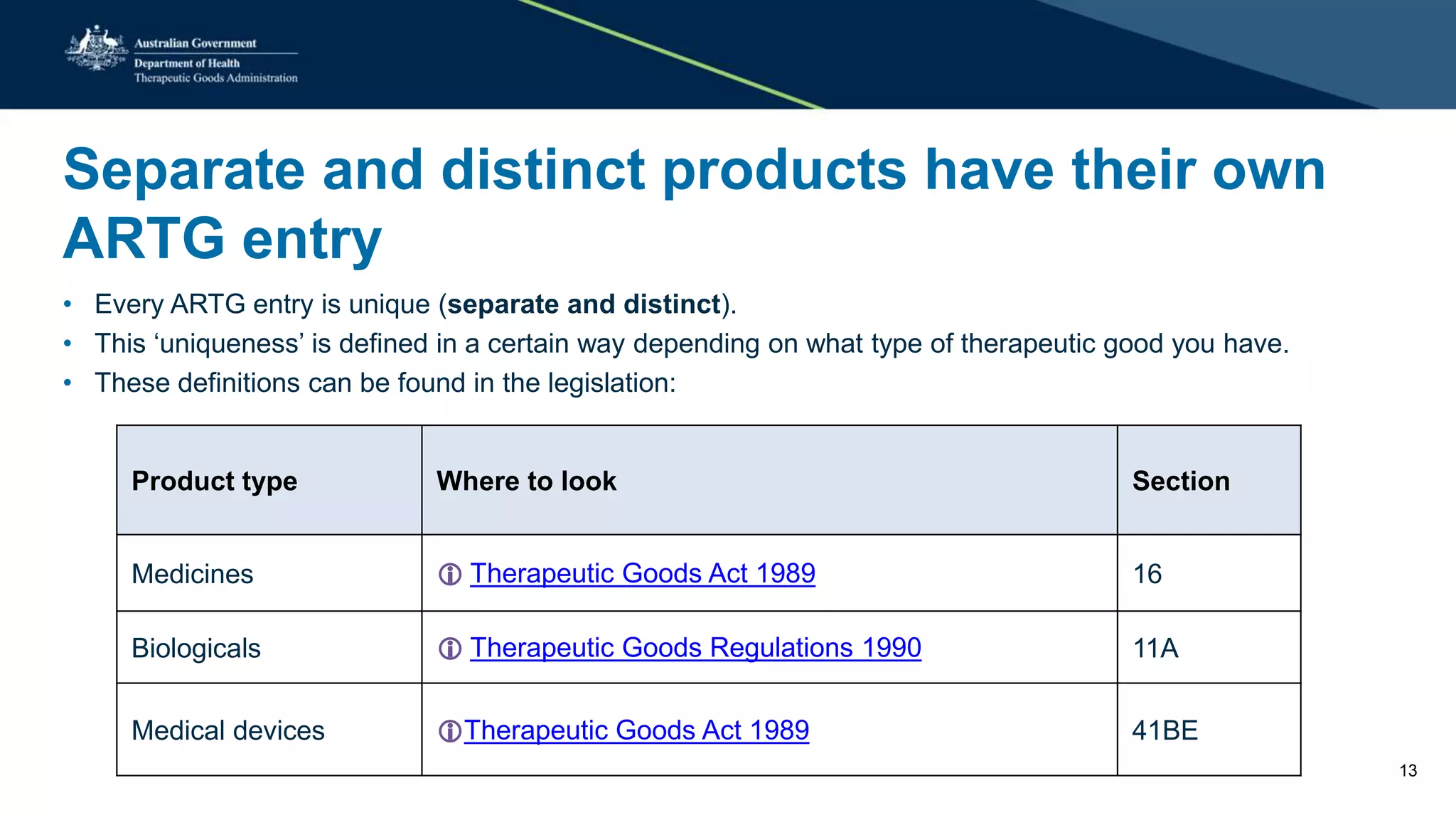
The document outlines the SME Assist program launched by the TGA to help small to medium enterprises navigate the regulatory landscape for therapeutic goods in Australia, initiated due to challenges identified in a 2016 review. Key features include guidance articles, interactive tools, tailored support, and upcoming events aimed at educating SMEs about their regulatory obligations. Since its launch, SME Assist has engaged a substantial audience with numerous inquiries, workshops, and partnerships to enhance accessibility and understanding of regulations.