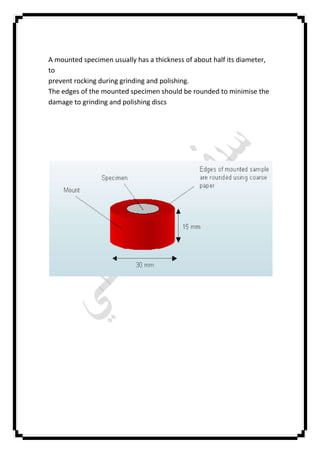
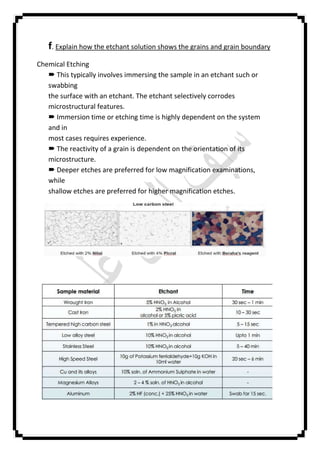
The document describes an experiment conducted at Baghdad University on preparing a metal specimen for microscopic examination, focusing on aspects such as grain size, types, and possible phase changes. It outlines the preparation process, including grinding and polishing techniques, as well as the use of etching solutions to reveal microstructural features. Additionally, it compares metallography with metallurgy and discusses the importance of temperature control and specimen rotation during the preparation process.