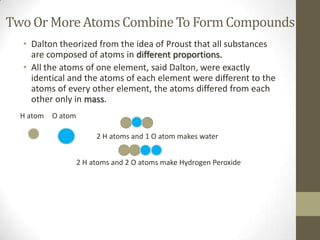
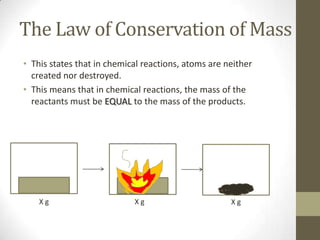
The document discusses the historical development of atomic theory, starting from Democritus's idea of the 'atomos' to John Dalton's postulates about atoms being the smallest indivisible particles that combine to form compounds. It highlights key contributions from various scientists, including Lavoisier's law of conservation of matter and Proust's law of definite composition, while also addressing the modern understanding of atoms and elementary particles. Dalton's theory, although foundational, is noted to have flaws, particularly regarding the indivisibility of atoms.